Review Article
VOLUME: 38 | ISSUE: 4 | Dec 30, 2022 | PAGE: (193 - 197) | DOI: 10.24911/BioMedica/5-857
Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways
Authors:
Nida Rasheed
, Sarah Ghafoor
Article Info
Authors
Nida Rasheed
M. Phil. Student, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan.
Sarah Ghafoor
Professor & Head, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan.
Publication History
Received: August 24, 2022
Revised: November 03, 2022
Accepted: December 05, 2022
Published: December 30, 2022
Abstract
Oral Squamous Cell Carcinoma (OSCC) has become prevalent worldwide and is one of the leading cause of death. Derangement of anti-apoptotic proteins is important regarding OSCC development. Over-expression of these proteins lead to prolonged cellular survival, thus increasing the susceptibility of tumor formation. Commiphora wightii and molmol are the natural herbs with ability to down-regulate anti-apoptotic proteins by acting on Nuclear Factor-kappa B (NF-κB) pathway, p53 pathway, signal transducer and activator of transcription pathway, estrogen, cyclooxygenase 2 and mitogen activated protein kinase pathway. NF-κB pathway has been found to involve in the release of cytokine storm associated with COVID-19 disease. Commiphora wightii and molmol suppress the COVID-19 infection by their anti-inflammatory and anti-viral properties. This review sheds light on the effect of Commiphora wightii and molmol on suppression of oral cancer and COVID-19 infection by modulation of anti-apoptotic proteins and inflammatory pathway.
Keywords: Anti-apoptotic, Commiphora molmol, Commiphora wightii, COVID-19, NF-κB, oral squamous cell carcinoma, tumor suppression
Pubmed Style
Nida Rasheed, Sarah Ghafoor. Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. BioMedica. 2022; 30 (December 2022): 193-197. doi:10.24911/BioMedica/5-857
Web Style
Nida Rasheed, Sarah Ghafoor. Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. https://biomedicapk.com/articles/online_first/857 [Access: July 27, 2024]. doi:10.24911/BioMedica/5-857
AMA (American Medical Association) Style
Nida Rasheed, Sarah Ghafoor. Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. BioMedica. 2022; 30 (December 2022): 193-197. doi:10.24911/BioMedica/5-857
Vancouver/ICMJE Style
Nida Rasheed, Sarah Ghafoor. Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. BioMedica. (2022), [cited July 27, 2024]; 30 (December 2022): 193-197. doi:10.24911/BioMedica/5-857
Harvard Style
Nida Rasheed, Sarah Ghafoor (2022) Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. BioMedica, 30 (December 2022): 193-197. doi:10.24911/BioMedica/5-857
Chicago Style
Nida Rasheed, Sarah Ghafoor. "Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways." 30 (2022), 193-197. doi:10.24911/BioMedica/5-857
MLA (The Modern Language Association) Style
Nida Rasheed, Sarah Ghafoor. "Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways." 30.December 2022 (2022), 193-197. Print. doi:10.24911/BioMedica/5-857
APA (American Psychological Association) Style
Nida Rasheed, Sarah Ghafoor (2022) Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways. , 30 (December 2022), 193-197. doi:10.24911/BioMedica/5-857
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 38(4):193-197
REVIEW ARTICLE
Commiphora wightii and molmol have therapeutic effects in oral cancers and COVID-19 disease by modulating anti-apoptotic proteins and inflammatory pathways
Nida Rasheed1, Sarah Ghafoor2* 
Received: 24 August 2022 Revised date: 03 November 2022 Accepted: 05 December 2022
Correspondence to: Sarah Ghafoor
*Professor and Head, Oral Biology Department, University of Health Sciences, Lahore, Pakistan.
Email: sarahghafoor@uhs.edu.pk
Full list of author information is available at the end of the article.
ABSTRACT
Oral squamous cell carcinoma (OSCC) has become prevalent worldwide and is one of the leading causes of death. Derangement of anti-apoptotic proteins is important regarding OSCC development. Over-expression of these proteins leads to prolonged cellular survival, thus increasing the susceptibility of tumor formation. Commiphora wightii and molmol are the natural herbs with ability to downregulate anti-apoptotic proteins by acting on nuclear factor-kappa B (NF-κB) pathway, p53 pathway, signal transducer and activator of transcription pathway, estrogen, cyclooxygenase 2, and mitogen-activated protein kinase pathway. NF-κB pathway has been found to involve in the release of cytokine storm associated with COVID-19 disease. Commiphora wightii and molmol suppress the COVID-19 infection by their anti-inflammatory and anti-viral properties. This review sheds light on the effect of C. wightii and molmol on suppression of oral cancer and COVID-19 infection by modulation of anti-apoptotic proteins and inflammatory pathway.
Keywords:
Oral squamous cell carcinoma, Commiphora wightii, Commiphora molmol, anti-apoptotic, tumor suppression, COVID-19, NF-κB.
Introduction
Cancers occur due to the increased expression of anti-apoptotic proteins and decreased expression of pro-apoptotic proteins. This results in uncontrolled cellular proliferation, ultimately leading to the lesions which can be malignant or benign. Among head and neck cancers, oral squamous cell carcinoma (OSCC) accounts for 90% of oral cancers and is considered among the leading causes of deaths in developing countries. It includes carcinoma of the lip, mouth, tongue, and oral cavity.1 The data collected regarding global burden caused by cancer over 28 years from 198 countries stated that Pakistan is the highest rank country in terms of incidence and mortality caused by OSCC.2 A study conducted from 2010 to 2019 in Karachi (Pakistan) reported that out of 22,858 registered cancer patients, 19.2% of cases belonged to lip and oral cancers and 97.7% of these cancers were OSCC.3 Another study conducted in the same region, to evaluate the pattern of oral malignancy, states that 63.8% of the patients with oral cancer belonged to the age group of 41-60 years and 96.6% of these patients were diagnosed with OSCC.4 Treatment modalities of cancer have side effects such as drug toxicity and intolerance to the treatment. Toxicity by chemotherapy and radiotherapy induced in the oral cavity includes oral mucositis, recurrent aphthous stomatitis, and xerostomia.5 Besides, the risk of toxicity, financial burden of these treatments on the patients limits their use. Thus, identification of cost-effective and natural agents with lesser side-effects is highly desirable for the treatment and prevention of oral cancers. Natural medicinal herbs may present as good candidates to treat cancerous conditions as they are easily accessible, less toxic, and economically viable. Commiphora wightii and Commiphora molmol are among the natural medicines that are found to exhibit tumor-inhibiting properties.6
COVID-19 is a respiratory disease caused by severe acute respiratory syndrome coronavirus. The first case of COVID-19 infection was identified in Wuhan region of China in December 2019. Since the start of pandemic, 399 million cases and 5.75 million deaths have been reported worldwide. Total number of cases reported in Pakistan is 1.47 million with 30,000 deaths reported till date.7 The symptoms caused by the disease include shortness of breath, fever, cough, loss of taste, and smell. COVID-19 infection mainly affects the lungs but other organ damage can also occur such as heart, kidneys, and brain.8
Commiphora wightii is a flowering plant from Burseraceae family. Commiphora wightii extract has been used as a medicine from centuries to treat many conditions including obesity, urinary complaints, liver disorders, arthritis, gastrointestinal diseases, malignant sores, ulcers, tumors, microbial infections, leukoderma, sinus, edema, and inflammation.9 The active ingredients of this herb constitute Z & E- guggulsterone, guggul lignans I & II, mukulol, gugglu tetrols, Z-guggulusterol, E-guggulusterol, allylcembrol, and c-27 guggulusterols I, II, and III. All of these active components contribute to the healing potential of C. wightii.10
Commiphora molmol (C.molmol/C.myrrha) also belongs to Burseraceae family like C. wightii.11 Commiphora molmol has been used in the remedy for mouth injuries and wounds.12 Additionally, C. molmol is used in the treatment of arthritis, digestive disorders, respiratory infection, leprosy, and syphilis and various parasitic, fungal, and bacterial infections. The active ingredients of C. molmol include curzerene, curzerenone, flavonoids, tannins, terpenoids, quinines, dipentene, caryophyllene oxide, α-pinene, furanoeudesma 1,3- diene, limonene, lindestrene, quercetin, and menthofuran. All these constituents of C. molmol act against microbes, viruses, parasites, and fungal infections.13
Electronic literature databases such as PubMed and Google Scholar were searched using the Medical subject headings terms such as cancer etiology, oral cancer, anti-apoptotic proteins, Commiphora wightii and Commiphora molmol, anti-apoptotic proteins, COVID-19 infection, and NF-κB in various combinations. All papers in English language having access of full text have been included in the study. Research papers published in language other than English and papers with limited access have been excluded from the study. This narrative review is focused on up to date knowledge of the role of C. wightii and molmol on oral cancer suppression through anti-apoptotic proteins and the effect of these medicinal herbs on COVID-19 infection through their action on NF-κB pathway.
Commiphora wightii and molmol in cancer therapy
A study conducted to evaluate the effects of C. wightii on cancer cells states that C. wightii inhibits tumor cell growth in vitro. Additionally, C. wightii caused a decrease in the growth kinetics of tumor cells. The research claimed that C. wightii increased the apoptosis of cells in OSCC by 27% by increasing the levels of NF-κB protein and decreasing the levels of Cyclin D1 protein in OSCC by 80%.14 Commiphora wightii modulates the gene products involved in metastasis of tumor cells by NF-Κb pathway, p53 pathway, signal transducer and activator of transcription 3 pathway, Ak strain transforming pathway, estrogen, mitogen-activated protein kinase pathway, Matrix metalloprotenase-9, and receptors (androgen & glucocorticoids).15 Commiphora molmol exhibit its anti-tumor potential as it increases the release of cytokine interferon gamma. In addition, C. molmol inhibited Proliferating Cell Nuclear Antigen expression, cyclooxygenase 2 (COX-2), and B cell lymphoma-2 (Bcl-2), thus facilitating apoptosis of cells which is believed a probable mechanism of cancer suppression.16
Commiphora wightii and molmol in relation to anti-apoptotic proteins
Commiphora wightii and molmol decrease the levels of anti-apoptotic proteins and protect against cancer development. Anti-apoptotic proteins are responsible for the prolonged cell survival and present as a potent candidate in the initiation, maintenance, and progression of carcinogenesis in the body by causing inhibition of cellular apoptosis. These proteins belong to B-cell lymphoma (Bcl) family. Anti-apoptotic proteins are Bcl-2, B cell lymphoma extra-large (Bcl-XL), B cell lymphoma W (Bcl-W), Myeloid leukemia 1 (Mcl-1), and anti-apoptotic protein A1.17
Commiphora wightii and molmol downregulate anti-apoptotic proteins of Bcl family and promote cancer suppression. Bcl-2 is an anti-apoptotic protein which increases the survival of cell. Increased expression of Bcl-2 proteins along with other factors (which increase cytotoxic tendency) leads toward development of cancer.18,19 Bcl-XL is involved in cellular migration and mitochondrial metabolism in all the cells of human body.20 An increased expression of this protein may lead to the onset of carcinogenesis. A study conducted to evaluate the association of C. wightii and anti-apoptotic proteins state that Bcl-2 and Bcl-XL levels were raised in the cells of human prostate cancerous tissue during the initial treatment with guggulsterone, however, the levels reduced markedly after 16-24 hour treatment with guggulsterone.21 Another study related the expression of COX2 with Bcl-XL and explained that both of these molecules work synergistically. COX-2 stimulates cancer stem cell by developing resistance to apoptosis, angiogenesis, inflammation, proliferation, invasion, and ultimately metastasis of cancer cells. Similarly, Bcl-XL promotes tumor growth by working with COX2 enzyme side by side.22 Commiphora molmol induces apoptosis of cancer cells by downregulating COX2 enzyme.16 Bcl-W is similar to Bcl-XL both in structure and function. Increased expression of Bcl-W contributes to decreased cellular death under cytotoxic conditions.23 Commiphora molmol consists of sesquiterpene compounds as one of its active ingredients which exhibit their anti-cancer property by downregulating Bcl-W along with survivin and heat shock proteins which induced DNA fragmentation and G0/G1-phase arrest and enhanced the intracellular Ca2+ concentration. Through these mechanisms, C. molmol causes downregulation of Bcl-W protein and ultimately lead to cellular apoptosis of cancerous cells.24 Commiphora wightii promotes cell death in a cancerous tissue by reducing the level of Mcl-1 with the help of its active components, terpenoids, and guggulsterone. Mcl-1 is an anti-apoptotic protein which promotes cell survival by interfering with the cellular pathway resulting in increased cytochrome c release from mitochondria. The cleavage of Mcl-1 leads to apoptosis of the cell, whereas its over-expression causes carcinogenesis and metastasis.25 A1 is an anti-apoptotic protein which prolongs cell survival by acting on mitogen-activated protein. A1 overexpression has been noticed in malignancies such as acute myeloid leukemia, melanoma, and lymphoma.26 Guggulsterone present in C. wightii downregulate the expression of anti-apoptotic proteins in tumor cells including oral cancers and liver cancers.27
Effects of C. molmol and wightii on COVID-19 disease
COVID-19 disease is a viral infection which involves various organs especially upper respiratory tract, kidneys, digestive, heart, and nervous system. The infection results in a surge of cytokines release such as NF-κB, interleukins (IL), leukotrienes (LT-α and LT-β), tumor necrosis factor, and granulocyte monocyte colony-stimulating factor.28 NF-κB is a pivotal pathway in inflammation and increase in COVID-19 infection leads to elevated levels of NF-κB. Excessive activation of NF-κB pathway leads to increased production of cytokines and chemokine, ultimately resulting in cytokine release syndrome.29 COVID-19 virus enters the human body by binding to its receptors such as angiotensin-converting enzyme 2, CD147 and transmembrane serine protease 2. The virus further undergo cleavage of glycoprotein in the viral envelop to efficiently enter the host body. As a result, p50 and p65 molecules of NF-κB pathway are released and get translocated from the cytoplasm into the nucleus to induce transcription of pro-inflammatory proteins in an infected cell. This inflammatory condition is further worsened by the activation of T cells (CD4 and CD8) in response to cytokines.30 This sequence of events leading to inflammatory state in response to cytokines can be prevented by guggulsterone, an active component of C. wightii, as shown in Figure 1.31 In murine cell lines, guggulsterone inhibits the activation of pro-inflammatory cytokines such as NF-κB pathway.32 Hence, it may contributes as an anti-inflammatory agent in COVID-19 infection similar to other inflammatory diseases such as Crohn’s disease and ulcerative colitis.33
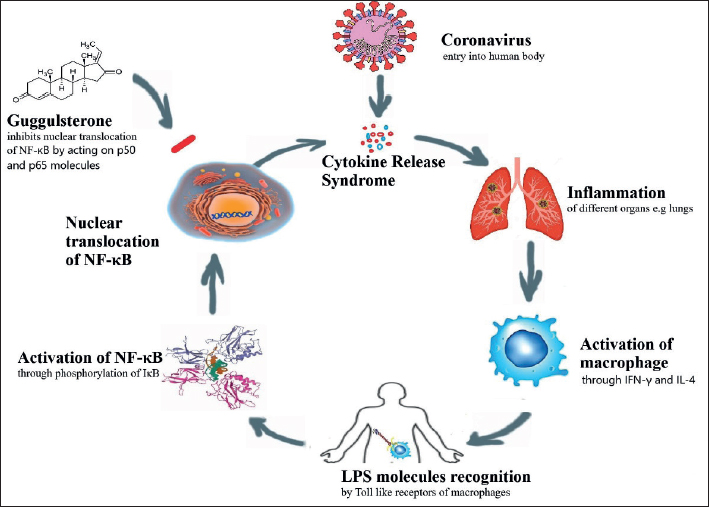
Figure 1. Association of guggulsterone with COVID-19 through NF-kB. Guggulsterone exhibits its anti-inflammatory activity by reducing the activation of NF-kB. Guggulsterone reduce NF-kB by blocking inhibitor of nuclear factor (IkB)
C. molmol is found beneficial in viral infections such as influenza A virus and Newcastle virus. A study conducted in 2021 on the cell lines of human epithelial carcinoma infected with influenza A virus concluded that C. molmol oil exhibit anti-viral activity by its most active components, curzerene and furanodienone. These components inhibit viral infection by inhibiting the replication and adsorption of virus to the cell surface.34 The essential oil of C. molmol was tested for its anti-viral activity against Newcastle virus. An aliquot of 0.1 ml of the viral suspension treated with C. molmol extract was inoculated in chicken embryos of 9 days old. The results showed anti-viral activity in the pure herbal oil as well as ethanol herbal oil.35 Hence, C. molmol might be helpful to combat viral infection caused by COVID-19 through its anti-viral property.
Conclusion
Anti-apoptotic proteins are critical in the survival of viable cells, however, their over expression leads to carcinogenesis. Commiphora wightii and molmol acts to slow down or even completely halt the development and progression of cancer formation by modulating anti-apoptotic proteins. Moreover, C. wightii and molmol have anti-inflammatory properties which reduce the severity of COVID-19 infection through NF-κB pathway.
Acknowledgement
The authors would like to acknowledge the higher education commission for providing e-library access to University of Health Sciences Lahore for database retrieval.
List of Abbreviations
| Bcl-2 | B cell lymphoma-2 |
| Bcl-W | B cell lymphoma W |
| Bcl-XL | Extra-large |
| COX-2 | Cyclooxygenase 2 |
| IL | Interleukins |
| MAPK | Mitogen activated protein kinase |
| Mcl-1 | Myeloid leukemia 1 |
| NF-κB | Nuclear factor-kappa B |
| OSCC | Oral squamous cell carcinoma |
Conflict of interest
None to declare.
Grant support and financial disclosure
The manuscript is extracted from M. Phil studies of NR that are funded by the University of Health Sciences Lahore, Pakistan.
Ethical approval
Not applicable.
Authors’ contributions
NR: Acquisition of published data and manuscript writing.
SG: Conception of study, critical revisions through intellectual content, and final approval of the manuscript.
Authors’ Details
Nida Rasheed1, Sarah Ghafoor2
- M. Phil. Student, Oral Biology Department, University of Health Sciences, Lahore, Pakistan
- Professor and Head, Oral Biology Department, University of Health Sciences, Lahore, Pakistan
References
- Feller L, Lemmer J. Oral squamous cell carcinoma: epidemiology, clinical presentation and treatment. J Cancer Ther. 2012;03(04):263–8. http:/dx.doi.org/10.4236/jct.2012.34037
- Ren ZH, Hu CY, He HR, Li YJ, Lyu J. Global and regional burdens of oral cancer from 1990 to 2017: results from the global burden of disease study. Cancer Commun (Lond). 2020;40(2-3):81–92. https://doi.org/10.1002%2Fcac2.12009
- Qureshi MA, Syed SA, Sharafat S. Lip and oral cavity cancers (C00-C06) from a mega city of Pakistan: ten-year data from the dow cancer registry. J Taibah Univ Med Sci. 2021;16(4):624–7. https://doi.org/10.1016/j.jtumed.2021.02.001
- Memon IM, Iqbal SM, Hussain SI, Baig MN. Pattern of oral malignancies at tertiary care hospitals. Pak J Surg. 2014;30(3):268–71.
- Zhang Q-Y, Wang F-X, Jia K-K, Kong L-D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front Pharmacol. 2018;9:1253. https://doi.org/10.3389/fphar.2018.01253
- Kunnumakkara AB, Banik K, Bordoloi D, Harsha C, Sailo BL, Padmavathi G, et al. Googling the Guggul (Commiphora and Boswellia) for prevention of chronic diseases. Front Pharmacol. 2018;9:686. https://doi.org/10.3389/fphar.2018.00686
- WHO coronavirus disease (COVID-19) dashboard. [cited 2022 Feb]. Available from: https://covid19.who.int/.
- Ciotti M, Ciccozzi M, Terrinoni A, Jiang W-C, Wang C-B, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci. 2020;57(6):365–88. https://doi.org/10.1080/10408363.2020.1783198
- Jaiswal S, Bara J, Soni R, Saksena P. Medical uses of Commiphora wightii. IOSR J Nurs Health Sci. 2016;5:76–81.
- Bhardwaj M, Alia A. Commiphora wightii (Arn.) Bhandari. Review of its botany, medicinal uses, pharmacological activities and phytochemistry. J Drug Del Ther. 2019;9(4-s):613–21. https://doi.org/10.22270/jddt.v9i4-s.3256
- Bhattacharjee MK, Alenezi T. Antibiotic in myrrh from Commiphora molmol preferentially kills nongrowing bacteria. Future Sci OA. 2020;6(4):FSO458. https://doi.org/10.2144%2Ffsoa-2019-0121
- Haffor A-SA. Effect of myrrh (Commiphora molmol) on leukocyte levels before and during healing from gastric ulcer or skin injury. J Immunotoxicol. 2010;7(1):68–75. https://doi.org/10.3109/15476910903409835
- Perveen K, Bokhari NA, Siddique I, Al-Rashid SA. Antifungal activity of essential oil of Commiphora molmol oleo gum resin. J Essent Oil Bear Plants. 2018;21(3):667–73. https://doi.org/10.1080/0972060X.2018.1492975
- Bharti V, Gupta UD, Das SN. Commiphora mukul extract and guggulsterone exhibit antitumour activity trough inhibition of Cyclin D1, BFK and induction of apoptosis in oral cancer cells. Asian J Pharm Clin Res. 2015;8:291–5.
- Shishodia S, Azu N, Rosenzweig JA, Jackson DA. Guggulsterone for Chemoprevention of Cancer. Curr Pharm Des. 2016;22(3):294–306. https://doi.org/10.2174/1381612822666151112153117
- Sun M, Hua J, Liu G, Huang P, Liu N, He X. Myrrh induces the apoptosis and inhibits the proliferation and migration of gastric cancer cells through down-regulating cyclooxygenase-2 expression. Bio Sci Rep. 2020;40(5). https://doi.org/10.1042/bsr20192372
- Han Z, Liang J, Li Y, He J. Drugs and clinical approaches targeting the antiapoptotic protein: a review. Biomed Res Int. 2019;2019:1212369. https://doi.org/10.1155%2F2019%2F1212369
- Mahmoud AM, Germoush MO, Al-Anazi KM, Mahmoud AH, Farah MA, Allam AA. Commiphora molmol protects against methotrexate-induced nephrotoxicity by up-regulating Nrf2/ARE/HO-1 signaling. Biomed Pharm Ther. 2018;106:499–509. https://doi.org/10.1016/j.biopha.2018.06.171
- Bharti V, Gupta UD, Das SN. Commiphora mukul extract and guggulsterone exhibit antitumour activity through inhibition of cyclin D1, NF-κβ and induction of apoptosis in oral cancer cells. Asian J Pharm Clin Res. 2015;8(4):291–5.
- Bessou M, Lopez J, Gadet R, Deygas M, Popgeorgiev N, Poncet D, et al. The apoptosis inhibitor Bcl-xL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production. Oncogene. 2020;39(15):3056–74. https://doi.org/10.1038/s41388-020-1212-9
- Singh SV, Zeng Y, Xiao D, Vogel VG, Nelson JB, Dhir R, et al. Caspase-dependent apoptosis induction by guggulsterone, a constituent of Ayurvedic medicinal plant Commiphora mukul, in PC-3 human prostate cancer cells is mediated by Bax and Bak. Mol Cancer Ther. 2005;4(11):1747–54. https://doi.org/10.1158/1535-7163.mct-05-0223
- Nardone G, Rocco A, Vaira D, Staibano S, Budillon A, Tatangelo F, et al. Expression of COX‐2, mPGE‐synthase1, MDR‐1 (P‐gp), and Bcl‐xL: a molecular pathway of H pylori‐related gastric carcinogenesis. J Pathol. 2004;202(3):305–12. https://doi.org/10.1002/path.1512
- Hartman ML, Czyz M. BCL-w: apoptotic and non-apoptotic role in health and disease. Cell Death Dis. 2020;11(4):1–16.
- Abu-Izneid T, Rauf A, Shariati MA, Khalil AA, Imran M, Rebezov M, et al. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol Res. 2020;161:105165. https://doi.org/10.1016/j.phrs.2020.105165
- Twilley D, Lall N. The role of natural products from plants in the development of anticancer agents. Nat Prod Drug Discov: Elsevier. 2018:139–78. https://doi.org/10.1016/B978-0-08-102081-4.00007-1
- Bittker JA, Weiwer M, Wei G, Germain A, Brown E, Dandapani S, et al. Discovery of inhibitors of anti-apoptotic protein A1. Probe reports from the NIH MLP [Internet]. 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK98916/
- Shishodia S, Harikumar KB, Dass S, Ramawat KG, Aggarwal BB. The guggul for chronic diseases: ancient medicine, modern targets. Anticancer Res. 2008;28(6A):3647–64.
- Zhang JH, Shangguan ZS, Chen C, Zhang HJ, Lin Y. Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-κB signaling pathway. Drug Des Devel Ther. 2016;10:1829–35. https://doi.org/10.2147%2FDDDT.S104602
- Su C-M, Wang L, Yoo D. Activation of NF-κB and induction of proinflammatory cytokine expressions mediated by ORF7a protein of SARS-CoV-2. Sci Rep. 2021;11(1):13464.
- Davies DA, Adlimoghaddam A, Albensi BC. The Effect of COVID-19 on NF-κB and neurological manifestations of disease. Mol Neurobiol. 2021;58(8):4178–87. https://doi.org/10.1007/s12035-021-02438-2
- Preethi L, Ganamurali N, Dhanasekaran D, Sabarathinam S. Therapeutic use of guggulsterone in COVID-19 induced obesity (COVIBESITY) and significant role in immunomodulatory effect. Obes Med. 2021;24:100346. https://doi.org/10.1016/j.obmed.2021.100346
- Zhang J-H, Shangguan Z-S, Chen C, Zhang H-J, Lin Y. Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-κB signaling pathway. Drug Des Devel Ther. 2016;10:1829. https://doi.org/10.2147%2FDDDT.S104602
- Mencarelli A, Renga B, Palladino G, Distrutti E, Fiorucci S. The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Bio Chem Pharm. 2009;78(9):1214–23. https://doi.org/10.1016/j.bcp.2009.06.026
- Madia VN, De Angelis M, De Vita D, Messore A, De Leo A, Ialongo D, et al. Investigation of Commiphora myrrha (Nees) Engl. Oil and Its Main Components for Antiviral Activity. Pharmaceuticals (Basel). 2021;14(3):243. https://doi.org/10.3390/ph14030243
- Gadir SA, Ahmed IM. Commiphora myrrha and commiphora Africana essential oils. J Chem Pharm Res. 2014;6(7):151–6.
