Original Article
VOLUME: 39 | ISSUE: 2 | Jun 25, 2023 | PAGE: (54 - 60) | DOI: 10.24911/BioMedica/5-886
Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs
Authors: Syeda Tahira Zaidi , Mahwash Malik , Javeria Sarfraz , Sadia Chiragh
Article Info
Authors
Syeda Tahira Zaidi
Senior Demonstrator, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan.
Mahwash Malik
Associate Professor, Department of Pharmacology, Central Park Medical College, Lahore, Pakistan.
Javeria Sarfraz
Assistant Professor, Department of Pharmacology, King Edward Medical University, Lahore, Pakistan.
Sadia Chiragh
Professor, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan.
Publication History
Received: December 14, 2022
Revised: April 24, 2023
Accepted: June 10, 2023
Published: June 25, 2023
Abstract
Background and Objective: Berberine has anti-inflammatory properties through pathways that are also involved in asthma. Therefore, it is postulated that it will be effective in reducing airway inflammation in the allergic asthmatic model. The objective of the current study was to observe the effects of berberine on the histopathology of lungs in sensitized Guinea pigs and compare the changes with that of the standard drug, dexamethasone.
Methods: This experimental study was conducted at the Postgraduate Medical Institute, Lahore, Pakistan, from February 2016 to April 2016. Twenty-four healthy Guinea pigs were divided randomly into four groups: normal control, Ovalbumin (OVA ) group, OVA + berberine group, and OVA + dexa group. The last three groups were sensitized on days 0 and 14 and challenged on days 25, 26, and 27 with OVA. Berberine and dexamethasone were administered intraperitoneally to the respective groups before each challenge. The animals were then sacrificed on day 28 under anesthesia, lungs were dissected, and tissue samples were assessed microscopically for morphology and status of inflammation in each group. Data were analyzed by applying the Kruskal-Wallis analysis of variance test followed by the Mann Whitney U test using SPSS 20.
Results: The comparison of histopathological changes revealed that the infiltration of inflammatory cells in the lung airways was significantly higher (p-value ≤0.01) in the OVA group as compared to the normal control group. Both OVA + berberine and OVA + dexa groups showed a reduction in total lung inflammation (p-value ≤0.01 vs. OVA group) equally. The effect of berberine on epithelial changes was nonsignificant (p-value 0.206 vs. OVA group), while dexamethasone showed significant improvement as compared to the OVA group (p-value 0.007). However, the difference between berberine and dexamethasone treatment was nonsignificant (p-value ≤0.05).
Conclusion: It was concluded that berberine improved the histopathological score of lung inflammation in sensitized Guinea pigs, equivalent to that of dexamethasone, with lesser effects on epithelial changes.
Keywords: Berberine, dexamethasone, asthma, airway inflammation, lung inflammation score.
Pubmed Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh. Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs. BioMedica. 2023; 25 (June 2023): 54-60. doi:10.24911/BioMedica/5-886
Web Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh. Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs. https://biomedicapk.com/articles/online_first/886 [Access: July 27, 2024]. doi:10.24911/BioMedica/5-886
AMA (American Medical Association) Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh. Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs. BioMedica. 2023; 25 (June 2023): 54-60. doi:10.24911/BioMedica/5-886
Vancouver/ICMJE Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh. Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs. BioMedica. (2023), [cited July 27, 2024]; 25 (June 2023): 54-60. doi:10.24911/BioMedica/5-886
Harvard Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh (2023) Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs. BioMedica, 25 (June 2023): 54-60. doi:10.24911/BioMedica/5-886
Chicago Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh. "Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs." 25 (2023), 54-60. doi:10.24911/BioMedica/5-886
MLA (The Modern Language Association) Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh. "Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs." 25.June 2023 (2023), 54-60. Print. doi:10.24911/BioMedica/5-886
APA (American Psychological Association) Style
Syeda Tahira Zaidi, Mahwash Malik, Javeria Sarfraz, Sadia Chiragh (2023) Berberine Improves Histopathological Changes of Allergic Airway Inflammation in Guinea Pigs. , 25 (June 2023), 54-60. doi:10.24911/BioMedica/5-886
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 39(2):54-60
ORIGINAL ARTICLE
Berberine improves histopathological changes of allergic airway inflammation in Guinea pigs
Syeda Tahira Zaidi1*, Mahwash Malik2, Javeria Sarfraz3, Sadia Chiragh4
Received: 14 December 2022 Revised date: 24 April 2023 Accepted: 10 June 2023
Correspondence to: Syeda Tahira Zaidi
*Senior Demonstrator, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan.
Email: drsyedatahira@yahoo.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Berberine has anti-inflammatory properties through pathways that are also involved in asthma. Therefore, it is postulated that it will be effective in reducing airway inflammation in the allergic asthmatic model. The objective of the current study was to observe the effects of berberine on the histopathology of lungs in sensitized Guinea pigs and compare the changes with that of the standard drug, dexamethasone.
Methods:
This experimental study was conducted at the Postgraduate Medical Institute, Lahore, Pakistan, from February 2016 to April 2016. Twenty-four healthy Guinea pigs were divided randomly into four groups: normal control, Ovalbumin (OVA ) group, OVA + berberine group, and OVA + dexa group. The last three groups were sensitized on days 0 and 14 and challenged on days 25, 26, and 27 with OVA. Berberine and dexamethasone were administered intraperitoneally to the respective groups before each challenge. The animals were then sacrificed on day 28 under anesthesia, lungs were dissected, and tissue samples were assessed microscopically for morphology and status of inflammation in each group. Data were analyzed by applying the Kruskal-Wallis analysis of variance test followed by the Mann Whitney U test using SPSS 20.
Results:
The comparison of histopathological changes revealed that the infiltration of inflammatory cells in the lung airways was significantly higher (p-value ≤0.01) in the OVA group as compared to the normal control group. Both OVA + berberine and OVA + dexa groups showed a reduction in total lung inflammation (p-value ≤0.01 vs. OVA group) equally. The effect of berberine on epithelial changes was nonsignificant (p-value 0.206 vs. OVA group), while dexamethasone showed significant improvement as compared to the OVA group (p-value 0.007). However, the difference between berberine and dexamethasone treatment was nonsignificant (p-value ≤0.05).
Conclusion:
It was concluded that berberine improved the histopathological score of lung inflammation in sensitized Guinea pigs, equivalent to that of dexamethasone, with lesser effects on epithelial changes.
Keywords:
Berberine, dexamethasone, asthma, airway inflammation, lung inflammation score.
Introduction
Asthma has been reported as the second most common cause of respiratory system-related deaths worldwide. By 2020, it has affected about 262 million people and likely caused 461,000 deaths globally.1,2 The prevalence of asthma is also very high in Pakistan.3 Since it is considered a threat and challenge to the immune system, it triggers multiple cellular immune responses. It alters the structural features and responses of airway cells, e.g., epithelial, endothelial, and smooth muscle cells.4 The treatment of asthma mainly includes anti-inflammatory drugs, especially corticosteroids, β2 agonists, bronchodilators, leukotriene receptor antagonists, biologics, and mast cell stabilizers.5
Corticosteroids are anti-inflammatory agents that effectively improve the symptoms of asthmatic patients and also arrest the progression of the disease. However, their use is associated with local and systemic adverse effects, especially in the case of prolonged use in chronic asthma. Adverse effects of drugs are less with the use of inhalers, but the socioeconomic status of most patients is the barrier to continuation of the treatment.6 Given these facts, there is a need to develop a better and more effective drug with minimum cost and adverse effects.
Asthma and allergy are lifelong diseases, and herbs are extremely effective. A large number of medicinal plants have been scientifically shown to have anti-asthmatic properties and are traditionally being used for the treatment of asthma. Even the currently used drugs, such as anticholinergics, methylxanthines, and cromolyn, have an herbal origin.7
A chemical substance called berberine is derived from the barks, rhizomes, and roots of numerous botanicals, primarily those in Asia, such as Berberis aristata, also known as the tree turmeric, and Berberis vulgaris, the Barberry plant. In Pakistan, Gilgit Baltistan and Central Karakorum National Park are the main areas where Berberis brondisian and Berberis pseud umbellate are found. It is also possible to synthesize berberine as chloride and sulfate salts.8 Berberine has been studied extensively and found to be useful in neoplastic, neurological, metabolic, and cardiovascular diseases. Most of the studies are preclinical, and multiple mechanisms have been suggested, including antioxidant, antihyperlipidemic, vasorelaxant, anti-inflammatory, and most importantly, cytotoxic.9 To find better therapeutic medicines for curing and preventing asthma, it may be helpful to block the nuclear factor kappa light chain enhancer of the activated B cells (NF-kB) pathway, as this pathway is involved in the pathogenesis of asthma.10 Berberine, prevents the NF-kB signaling, leading to reduced production of thymic stromal lymphopoietin (TSLP) and subsequent inflammation.11 The aim of the present research was to compare the anti-inflammatory effects of dexamethasone and berberine on histopathology of the lung tissue in the sensitized Guinea pigs.
Methods
A total of 24 Guinea pigs that were in good health, of either gender and weighing between 340 and 500 g, were habituated to a temperature range of 22°C to 24°C for acclimatization for a week at the Animal Experimental Laboratory of the Department of Pharmacology, Post Graduate Medical Institute Lahore, Pakistan. With unrestricted access to water and food, the animals were housed in a setting with natural day and night cycles. The Foundation’s Basic Sciences Research Ethical Committee approved the experiment.
The lottery method was used to assign 24 animals into 4 groups. During the study, Ovalbumin (OVA) (Sigma Aldrich, Poole UK), aluminum hydroxide (alum) (Bio sector, Denmark), phosphate buffer saline (PBS) (Sigma Aldrich, Germany), berberine chloride (Sigma, USA), dexamethasone (OBS Pharm, Pakistan), Hematoxylin and Eosin stain (Medline diagnostic, Lahore, Pakistan) were used (Table 1).
Airway inflammation was induced by employing standard methodology12 in animals of three groups, excluding the normal control group (Figure 1).
Lung histopathology
The animals were sacrificed under chloroform anesthesia, the lungs were dissected, and cassettes were made by fixing them for 24 hours in a 10% formalin solution. Following the tissue processing, samples were cut into 5 µm sections, mounted on glass slides, and stained with eosin and hematoxylin to determine the lung morphology and status of inflammation.
Lung inflammation consisting of alveolar septal, perivascular, or peribronchial infiltrates was categorized on a severity scale15,16 as shown below,
Score 0 = No inflammation
Score 1 = Few scattered inflammatory cells
Score 2 = Larger areas of bronchi or vessels surrounded by one layer of inflammatory cell infiltration
Score 3 = Larger areas of bronchi or vessels surrounded by 2-4 layers of inflammatory cell infiltrationlayer
Score 4 = Larger areas of bronchi or vessels surrounded by thick layer (>4 layers) of inflammatory cell infiltration.
Peri-bronchial, alveolar septal, and perivascular inflammation levels for every animal were added to determine the total lung inflammation scores. Airway epithelial changes, including the destruction of ciliated cells, mucous goblet cell hyperplasia, squamous metaplasia, and so on, induced by the given drugs were also evaluated and scored by applying a severity scale.15,16
Table 1. Study protocol for preparation of model and treatment of groups.
| Group | Sensitization Day 0 and 14 Intraperitoneal |
Challenge Day 25-27 Inhalation |
Treatment Half an hour before each challenge Intraperitoneal |
|---|---|---|---|
| Normal control | PBS 1.5 ml | PBS | DW (5 ml/kg) |
| OVA group | OVA 0.5 ml (100 µg) + alum 1 ml (200 mg) in PBS12 | 1% OVA in PBS12 | DW (5 ml/kg) |
| OVA + Berberine | OVA 0.5 ml (100 µg) + alum 1 ml (200 mg) in PBS | 1% OVA in PBS | Berberine in DW (1.8 mg/kg)13 |
| OVA + Dexa | OVA 0.5 ml (100 µg) + alum 1 ml (200 mg) in PBS | 1% OVA in PBS | Dexamethasone in DW (20 mg/kg)14 |
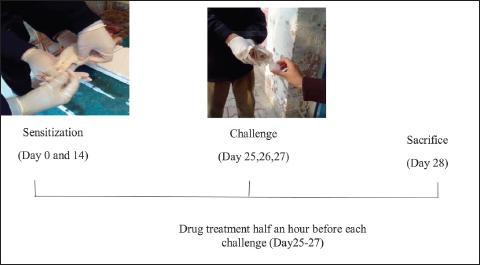
Figure 1. Protocol flow diagram of the study.
Statistical analysis
Data were entered and analyzed by using SPSS 20. The frequencies and probability of the histological scores for inflammatory conditions and epithelial changes were documented. Furthermore, the Kruskal-Wallis analysis of variance (ANOVA) was utilized to compare the findings between groups. The Mann-Whitney U test was performed for post hoc analysis.
Results
The histological scores of lung inflammation and epithelial changes in Guinea pigs of different study groups are presented in Table 2.
Perivascular, peribronchial, alveolar septal infiltrates, and bronchial epithelial changes were absent in the normal control group. In the OVA group, perivascular, peribronchial, and alveolar septal infiltrates, and epithelial changes, including the destruction of ciliated cells, mucous goblet cell hyperplasia, and/or squamous metaplasia, were moderate to severe. However, in OVA + berberine group, these changes were minimal to moderate, while minimal to mild in OVA + dexa group.
Histopathological changes were compared by taking the mean ranks of each pathological change, and the Kruskal Wallis ANOVA Test was applied, which showed statistically significant findings. The post hoc comparison of histopathological changes revealed that inflammatory cell infiltration was significantly higher in the OVA group as compared to the normal control group. OVA + berberine and OVA + dexa groups showed significant effects on all variables of inflammation, but the effect of berberine on epithelial changes was nonsignificant, as shown in Table 3.
Eventually, a p-value of <0.001 was observed in the comparison of the composite score for overall lung inflammation comparing groups. Dexamethasone, having the lowest overall rank, was substantially different from the OVA group (p = 0.003) and not statistically different from berberine (p = 0.118). In addition, the berberine’s mean rank was much lower than the OVA group, with a p-value of 0.004. The mean ranks for total lung inflammation among the four study groups are shown in Figure 3.
Discussion
Individuals are searching for substitute herbal medications that are harmless, affordable, and medically proven because the therapy for asthma is not without side effects and is often ineffective for many individuals.17 An experimental model of induced asthma was developed in the Guinea pigs to observe the useful effect of berberine on airway inflammation. Improvement in lung inflammation equivalent to dexamethasone was observed with berberine treatment on histological examination of lung tissue, while improvement in epithelial damage was less marked.
The study comprised four groups, a normal control group for reference. The remaining three groups were sensitized with OVA and alum. The OVA group animals were treated with distilled water as a placebo, drug control was treated with the standard drug dexamethasone for comparison, and the experimental group was treated with berberine. The OVA sensitization resulted in increased infiltration of inflammatory cells and caused epithelial changes in the airway mucosa. This is one of the most common methods to induce airway inflammation.18
Among the four groups, histological indicators of lung inflammation were studied and compared. When compared to the standard control group, the OVA group’s histological evaluation showed inflammatory cell infiltration and noticeable epithelial alterations with p-values <0.01, which is in concordance with the findings of Vasconcelos et al.19
Table 2. Histological findings in the lungs of animals in each group.
| Lesion score | Normal control group | OVA group | OVA + Berberine group | OVA + Dexa group | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Perivascular infiltrates | 0 | 6 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) |
| 1 | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | 4 (66.7%) | |
| 2 | 0 (0.0%) | 0 (0.0%) | 4 (66.7%) | 1 (16.7%) | |
| 3 | 0 (0.0%) | 2 (33.3%) | 1 (16.7%) | 0 (0.0%) | |
| 4 | 0 (0.0%) | 4 (66.7%) | 0 (0.0%) | 0 (0.0%) | |
| Peribronchial infiltrates | 0 | 6 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) |
| 1 | 0 (0.0%) | 0 (0.0%) | 3 (50.0%) | 5 (83.3%) | |
| 2 | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) | 0 (0.0%) | |
| 3 | 0 (0.0%) | 2 (33.3%) | 1 (16.7%) | 0 (0.0%) | |
| 4 | 0 (0.0%) | 4 (66.7%) | 0 (0.0%) | 0 (0.0%) | |
| Alveolar septal infiltrates | 0 | 6 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 1 | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) | 2 (33.3%) | |
| 2 | 0 (0.0%) | 0 (0.0%) | 3 (50.0%) | 4 (66.7%) | |
| 3 | 0 (0.0%) | 1 (16.7%) | 1 (16.7%) | 0 (0.0%) | |
| 4 | 0 (0.0%) | 5 (83.3%) | 0 (0.0%) | 0 (0.0%) | |
| Epithelial changes | 0 | 6 (100.0%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) |
| 1 | 0 (0.0%) | 0 (0.0%) | 2 (33.3%) | 4 (66.7%) | |
| 2 | 0 (0.0%) | 4 (66.7%) | 3 (50.0%) | 1 (16.7%) | |
| 3 | 0 (0.0%) | 2 (33.3%) | 1 (16.7%) | 0 (0.0%) | |
Table 3. Group-wise comparison of mean ranks of perivascular, peribronchial, alveolar septal, and epithelial changes in all study groups by Mann-Whitney test.
| (I) Study groups | (J) Study groups | Perivascular infiltrates(p-value) | Peribronchial infiltrates (p-value) | Alveolar septal infiltrates (p-value) | Epithelial changes (p-value) |
|---|---|---|---|---|---|
| Normal control group | OVA group | 0.002** | 0.002** | 0.001*** | 0.002** |
| OVA + Berberine group | 0.002** | 0.002** | 0.002** | 0.002** | |
| OVA + Dexa group | 0.006** | 0.005** | 0.002** | 0.006** | |
| OVA group | OVA + Berberine group | 0.004** | 0.005** | 0.003** | 0.206 |
| OVA + Dexa group | 0.003** | 0.002** | 0.002** | 0.007** | |
| OVA + Berberine group | OVA + Dexa group | 0.025* | 0.045* | 0.715 | 0.067* |
When compared to the OVA group, Guinea pigs fed with berberine displayed considerably less airway inflammation (p-value 0.01) and better but statistically insignificant epithelial alterations (p-value = 0.206). In contrast, berberine treatment in a trial performed by Zhang et al.10 revealed a significantly reduced inflammation of the lung (p-value ≤ 0.05). Similar results were published by another study, which concluded that berberine significantly improved histopathological changes in the OVA-induced rat model of allergic rhinitis.20 When the effects of berberine and dexamethasone on total lung inflammation were compared, the findings of the current study revealed insignificant results as compared to that of other published studies where significant values were observed (p-value <0.01).21,22
The role of TSLP, Th2, and Th1 stimulated by NF-kB in human and animal asthmatic models is well documented. An important part of triggering, sustaining, and advancing allergic airway inflammation is the sequence-specific DNA-binding factor NF-kB.23 It is a component of regulatory I kappa B protein molecules (IKB) in the cytoplasm. When IKB is broken down by IKB kinase as a result of phosphorylation, the NF-kB passage is triggered, which causes the release of NF-kB dimers, thus triggering the production of NF-kB-dependent genes.24 TSLP is released, and Th 2 cells are activated through the NF-kB pathway.25 The focalization of asthma is significantly influenced by TSLP and Th 2 cells.26 Berberine is shown to inhibit the NF-kB pathway, which blocks the production of TSLP, with a resultant increase in Th1 cells and a decrease in Th2 cells.27,28
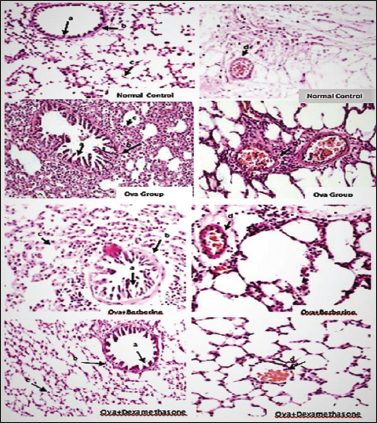
Figure 2. Photomicrograph of lung tissue section from different study groups; (a) bronchial, (b) peri bronchial, (c) alveolar septal, and (d) perivascular inflammation (H & E stain, 400×).

Figure 3. Bar chart showing mean ranks for total lung inflammation among four groups.
**p-value <0.01 versus OVA group, ++p-value <0.01 versus normal control group.
To conclude, the berberine and dexamethasone treatment groups showed equivalent effects on total lung inflammation, but the effect of berberine on epithelial changes was not significant. Hence, berberine is a potential candidate to reduce lung inflammation in asthmatic models.
Conclusion
Berberine improves lung inflammation in an experimental model of allergic airway inflammation equivalent to that of dexamethasone; however, its ameliorating effects on alveolar and bronchial epithelial cells are less marked as compared to dexamethasone.
Limitations of the Study
This study used a standard model of bronchial asthma, but there was a lack of observations regarding the effect on bronchial hyper-responsiveness, which is also responsible for symptoms of asthma. Therefore, the effects of berberine need to be studied on airway hyper-responsiveness as well before planning human studies for therapeutic application. The second limitation faced was that only one dose could be used due to the limited number of animals available. Dose-response curves for determining potency and maximal efficacy are pivotal in pharmacological experiments, which may be planned for future studies. Another recommendation is to observe the effect of co-administration with other anti-asthmatic medications.
Acknowledgement
The authors are thankful to Dr. Zakia Waheed, Assistant Professor, Department of Pathology, Fatima Memorial Hospital College of Medicine & Dentistry, Lahore, Pakistan, for supervising the histopathological analysis.
List of Abbreviations
| ANOVA | Analysis of variance |
| OVA | Ovalbumin |
| PBS | Phosphate buffer saline |
| TSLP | Thymic stromal lymphopoietin |
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose.
Ethical approval
Ethical approval was granted by the Institutional Ethics Committee of the Foundation’s Basic Sciences Research Ethical Committee vide Letter No: 00-19-S dated 15.06.2017.
Authors’ contributions
STZ: Conception of idea, acquisition, and analysis of data, drafting of manuscript.
MM: Interpretation of data, drafting of manuscript.
JS: Acquisition of data, drafting of manuscript.
SC: Conception of idea, critical intellectual input.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ Details
Syeda Tahira Zaidi1, Mahwash Malik2, Javeria Sarfraz3, Sadia Chiragh4
- Senior Demonstrator, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan
- Associate Professor, Department of Pharmacology, Central Park Medical College, Lahore, Pakistan
- Assistant Professor, Department of Pharmacology, King Edward Medical University, Lahore, Pakistan
- Professor, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan
References
- Mattiuzzi C, Lippi G. Worldwide asthma epidemiology: insights from the global health data exchange database. Int Forum Allergy Rhinol. 2020 Jan;10(1):75–80. https://doi.org/10.1002/alr.22464
- Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020 Oct;396(10258):1204–22.
- Razzaq S, Nafees AA, Rabbani U, Irfan M, Naeem S, Khan MA, et al. Epidemiology of asthma and associated factors in an urban Pakistani population: adult asthma study-Karachi. BMC Pulmo Med. 2018 [cited 2022 Sept 12];18(1):184–96. Available from: https://ecommons.aku.edu/pakistan_fhs_mc_chs_chs/749; https://doi.org/10.1186/s12890-018-0753-y
- King GG, James A, Harkness L, Wark PA. Pathophysiology of severe asthma: we’ve only just started. Respirology. 2018 Mar;23(3):262–71. https://doi.org/10.1111/resp.13251
- Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med. 2022 Jan;205(1):17–35. https://doi.org/10.1164/rccm.202109-2205PP
- Neffen H, Chahuàn M, Hernández DD, Vallejo-Perez E, Bolivar F, Sánchez MH, et al. Key factors associated with uncontrolled asthma - the asthma control in Latin America study. J Asthma. 2020 Feb;57(2):113–22. https://doi.org/10.1080/02770903.2018.1553050
- Alamgeer YW, Younis W, Asif H, Sharif A, Riaz H, Bukhari IA, et al. Traditional medicinal plants used for respiratory disorders in Pakistan: a review of the ethno-medicinal and pharmacological evidence. Chin Med. 2018 Sep;13(1):48. https://doi.org/10.1186/s13020-018-0204-y
- Kumar A, Ekavali, Chopra K, Mukherjee M, Pottabathini R, Dhull DK. Current knowledge and pharmacological profile of berberine: an update. Eur J Pharmacol. 2015 Aug;761:288–97. https://doi.org/10.1016/j.ejphar.2015.05.068
- Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020 Oct;14(5):564–82. https://doi.org/10.1007/s11684-019-0724-6
- Zhang T, Yang S, Du J, Jinfu Y, Shumin W. Platycodin D attenuates airway inflammation in a mouse model of allergic asthma by regulation NF-κB pathway. Inflammation. 2015;38(3):1221–8. https://doi.org/10.1007/s10753-014-0089-6
- Li Z, Zheng J, Zhang N, Li C. Berberine improves airway inflammation and inhibits NF-κB signaling pathway in an ovalbumin-induced rat model of asthma. J Asthma. 2016 Dec;53(10):999–1005. https://doi.org/10.1080/02770903.2016.1180530
- Kwon Y, Sohn S, Lee H, Shin M, Bae H. Electroacupuncture attenuates ovalbumin induced allergic asthma via modulating CD4+CD25+ regulatory T cells. Evid Based Complement Alternat Med. 2012;2012:647308. https://doi.org/10.1155/2012/647308
- Zaidi TS, Kausar R, Malik M, Sarfraz J, Shafiq A, Chiragh S. Comparison of berberine and dexamethasone on blood and bronchial inflammatory cells of ovalbumin sensitized Guinea pigs. Esculapio. 2021;17(01):34–8. https://doi.org/10.51273/esc21.251717
- Lowe AP, Thomas RS, Nials AT, Kidd EJ, Broadley KJ, Ford WR. Route of administration affects corticosteroid sensitivity of a combined ovalbumin and lipopolysaccharide model of asthma exacerbation in Guinea pigs. J Pharmacol Exp Ther. 2017 Aug;362(2):327–37. https://doi.org/10.1124/jpet.117.241927
- Moon KA, Kim SY, Kim TB, Yun ES, Park CS, Cho YS, et al. Allergen-induced CD11b+ CD11c(int) CCR3+ macrophages in the lung promote eosinophilic airway inflammation in a mouse asthma model. Int Immunol. 2007 Dec;19(12):1371–81. https://doi.org/10.1093/intimm/dxm108
- Nandedkar SD, Feroah TR, Hutchins W, Weihrauch D, Konduri KS, Wang J, et al. Histopathology of experimentally induced asthma in a murine model of sickle cell disease. Blood. 2008 Sep;112(6):2529–38. https://doi.org/10.1182/blood-2008-01-132506
- Amaral-Machado L, Oliveira WN, Moreira-Oliveira SS, Pereira DT, Alencar EN, Tsapis N, et al. Use of natural products in asthma treatment. Evid Based Complement Alternat Med. 2020 Feb;2020:1021258. https://doi.org/10.1155/2020/1021258
- Mincham KT, Scott NM, Lauzon-Joset JF, Leffler J, Stumbles PA, Holt PG, et. al. Early life ovalbumin sensitization and aerosol challenge for the induction of allergic airway inflammation in a BALB/c murine model. Bio Protoc. 2019;9(5):e3181. https://doi.org/10.21769/BioProtoc.3181
- Vasconcelos LH, Silva MD, Costa AC, de Oliveira GA, de Souza IL, Queiroga FR, et al. A Guinea pig model of airway smooth muscle hyperreactivity induced by chronic allergic lung inflammation: contribution of epithelium and oxidative stress. Front Pharmacol. 2019 Jan;9:1547. https://doi.org/10.3389/fphar.2018.01547
- Sakat MS, Kilic K, Kandemir FM, Yildirim S, Sahin A, Kucukler S, et al. The ameliorative effect of berberine and coenzyme Q10 in an ovalbumin-induced allergic rhinitis model. Eur Arch Otorhinolaryngol. 2018 Oct;275(10):2495–505. https://doi.org/10.1007/s00405-018-5104-3
- Antwi AO, Obiri DD, Osafo N. Stigmasterol modulates allergic airway inflammation in Guinea pig model of ovalbumin-induced asthma. Mediators Inflamm. 2017;2017:2953930. https://doi.org/10.1155/2017/2953930
- Antwi AO, Ekuadzi E, Boakye-Gyasi E, Agbettor ON, Opare CA, Atobrah R, et al. Pseudocedrela kotschyi limits airway inflammation in ovalbumin-induced asthma in Guinea pigs. Sci Afr. 2021;14:e01023. https://doi.org/10.1016/j.sciaf.2021.e01023
- Gregorczyk I, Maślanka T. Blockade of RANKL/RANK and NF-ĸB signalling pathways as novel therapeutic strategies for allergic asthma: a comparative study in a mouse model of allergic airway inflammation. Eur J Pharmacol. 2020 Jul;879:173129. https://doi.org/10.1016/j.ejphar.2020.173129
- Sengupta S, Haczku A. Targeting IκBNS in allergic asthma: where it resides, matters. Allergy. 2017 Jul;72(7):1003–5. https://doi.org/10.1111/all.13126
- Lamiable O, Mayer JU, Munoz-Erazo L, Ronchese F. Dendritic cells in Th2 immune responses and allergic sensitization. Immunol Cell Biol. 2020 Nov;98(10):807–18. https://doi.org/10.1111/imcb.12387
- Peebles RS Jr, Aronica MA. Proinflammatory pathways in the pathogenesis of asthma. Clin Chest Med. 2019 Mar;40(1):29–50. https://doi.org/10.1016/j.ccm.2018.10.014
- Li W, Yin N, Tao W, Wang Q, Fan H, Wang Z. Berberine suppresses IL-33-induced inflammatory responses in mast cells by inactivating NF-κB and p38 signaling. Int Immunopharmacol. 2019 Jan;66:82–90. https://doi.org/10.1016/j.intimp.2018.11.009
- Tew XN, Xin Lau NJ, Chellappan DK, Madheswaran T, Zeeshan F, Tambuwala MM, et al. Immunological axis of berberine in managing inflammation underlying chronic respiratory inflammatory diseases. Chem Biol Interact. 2020 Feb;317:108947. https://doi.org/10.1016/j.cbi.2020.108947
