Original Article
VOLUME: 39 | ISSUE: 2 | Jun 25, 2023 | PAGE: (78 - 83) | DOI: 10.24911/BioMedica/5-895
Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model
Authors:
Akfish Zaheer
, Rabab Miraj
, Sidra Mushtaq
, Qurat-tul-Ain
, Hafiz Muhammad Imran Aziz
, Sadia Chiragh
Article Info
Authors
Akfish Zaheer
Assistant Professor, Department of Pharmacology, Independent Medical College, Faisalabad, Pakistan.
Rabab Miraj
Assistant Professor, Department of Pharmacology, Sialkot Medical College, Sialkot, Pakistan.
Sidra Mushtaq
Assistant Professor, Department of Pharmacology, Independent Medical College, Faisalabad, Pakistan.
Qurat-tul-Ain
Assistant Professor, Department of Pharmacology, Pak Red Crescent Medical and Dental College, Lahore, Pakistan.
Hafiz Muhammad Imran Aziz
Associate Professor, Department of Pharmacology, ABWA Medical College, Faisalabad, Pakistan.
Sadia Chiragh
Head, Department of Pharmacology, Al-Aleem Medical and Dental College, Postgraduate Medical Institute, Lahore, Pakistan.
Publication History
Received: January 02, 2023
Revised: April 22, 2023
Accepted: June 08, 2023
Published: June 25, 2023
Abstract
Background and Objective: Canagliflozin reduces insulin resistance in diabetics and is hypothesized to produce a beneficial effect in polycystic ovarian syndrome (PCOS). Therefore, this study is planned to compare the effects of canagliflozin and metformin alone and in combination on ovarian histology of rat models with letrozole-induced PCOS.
Methods: It was a randomized experimental study on Sprague Dawley rats. A total of N = 40 female rats were divided randomly into six groups (A-F). With the exception of normal control group A, rats were given letrozole 1 mg/kg daily for 21 days till PCOS was induced. Group B was disease control, while rats in groups C-F were administered canagliflozin (10 mg/kg), metformin (100 mg/kg), a combination of canagliflozin (10 mg/kg) with metformin (100 mg/kg), and combination of canagliflozin (5 mg/kg) with metformin (50 mg/kg), respectively. Animals were sacrificed on the 48th day. Ovaries and uterus were removed, weighed, and processed for further histopathological analysis.
Results: All treatment groups showed significant improvement in ovarian histology. The number of primary and secondary follicles and cystic follicles was significantly lower (p < 0.001) in all treatment groups as compared to the disease control group.
Conclusion: Canagliflozin is effective for the treatment of PCOS and augments the effect of metformin in a rat model.
Keywords: Polycystic ovarian syndrome, canagliflozin, metformin, ovary, histology, treatment.
Pubmed Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh. Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model. BioMedica. 2023; 25 (June 2023): 78-83. doi:10.24911/BioMedica/5-895
Web Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh. Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model. https://biomedicapk.com/articles/online_first/895 [Access: July 27, 2024]. doi:10.24911/BioMedica/5-895
AMA (American Medical Association) Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh. Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model. BioMedica. 2023; 25 (June 2023): 78-83. doi:10.24911/BioMedica/5-895
Vancouver/ICMJE Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh. Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model. BioMedica. (2023), [cited July 27, 2024]; 25 (June 2023): 78-83. doi:10.24911/BioMedica/5-895
Harvard Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh (2023) Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model. BioMedica, 25 (June 2023): 78-83. doi:10.24911/BioMedica/5-895
Chicago Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh. "Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model." 25 (2023), 78-83. doi:10.24911/BioMedica/5-895
MLA (The Modern Language Association) Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh. "Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model." 25.June 2023 (2023), 78-83. Print. doi:10.24911/BioMedica/5-895
APA (American Psychological Association) Style
Akfish Zaheer, Rabab Miraj, Sidra Mushtaq, Qurat-tul-Ain, Hafiz Muhammad Imran Aziz, Sadia Chiragh (2023) Effect of Canagliflozin Alone and in Combination with Metformin on Ovarian Histology of a Polycystic Ovary Syndrome Rat Model. , 25 (June 2023), 78-83. doi:10.24911/BioMedica/5-895
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 39(2):78-83
ORIGINAL ARTICLE
Effect of canagliflozin alone and in combination with metformin on ovarian histology of a polycystic ovary syndrome rat model
Akfish Zaheer1*, Rabab Miraj2, Sidra Mushtaq1, Qurat-tul-Ain3, Hafiz Muhammad Imran Aziz4, Sadia Chiragh5
Received: 02 January 2023 Revised date: 22 April 2023 Accepted: 08 Jun 2023
Correspondence to: Akfish Zaheer
*Assistant Professor, Department of Pharmacology, Independent Medical College, Faisalabad, Pakistan.
Email: akfishzaheer80@gmail.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Canagliflozin reduces insulin resistance in diabetics and is hypothesized to produce a beneficial effect in polycystic ovarian syndrome (PCOS). Therefore, this study is planned to compare the effects of canagliflozin and metformin alone and in combination on ovarian histology of rat models with letrozole-induced PCOS.
Methods:
It was a randomized experimental study on Sprague Dawley rats. A total of N = 40 female rats were divided randomly into six groups (A-F). With the exception of normal control group A, rats were given letrozole 1 mg/kg daily for 21 days till PCOS was induced. Group B was disease control, while rats in groups C-F were administered canagliflozin (10 mg/kg), metformin (100 mg/kg), a combination of canagliflozin (10 mg/kg) with metformin (100 mg/kg), and combination of canagliflozin (5 mg/kg) with metformin (50 mg/kg), respectively. Animals were sacrificed on the 48th day. Ovaries and uterus were removed, weighed, and processed for further histopathological analysis.
Results:
All treatment groups showed significant improvement in ovarian histology. The number of primary and secondary follicles and cystic follicles was significantly lower (p < 0.001) in all treatment groups as compared to the disease control group.
Conclusion:
Canagliflozin is effective for the treatment of PCOS and augments the effect of metformin in a rat model.
Keywords:
Polycystic ovarian syndrome, canagliflozin, metformin, ovary, histology, treatment.
Introduction
Polycystic ovarian syndrome (PCOS) is a reproductive, metabolic, and psychological condition with adverse impact across the lifespan of females. The etiology is complex and includes genetic and epigenetic susceptibility, hypothalamic and ovarian dysfunction, excess androgen exposure, insulin resistance, and adiposity-related mechanisms.1
Typically, women with PCOS show clinical and biochemical hyperandrogenism, oligoanovulation, and micropolycystic morphology of the ovaries.2 Considering the heterogeneity of PCOS, treatment should be individualized and adapted to the specific needs of each patient.3 The biochemical hallmark of metabolic disturbances in PCOS is insulin resistance. Metformin reduces insulin resistance, decreases gonadotropin hormone-releasing hormone pulses from the pituitary, and causes a decrease in luteinizing hormone secretion from ovaries and theca cells. Moreover, it increases the sex hormone binding globulin, which causes a decrease in circulating androgen levels in the plasma.4 Perhaps among all available medications, metformin has been shown to be the safest. Yet, on the other hand, a meta-analysis conducted by Kojok et al.5 reports when used during assisted reproductive technology cycles, metformin co-treatment has not been demonstrated to enhance clinical pregnancies, miscarriages, or live births, but has been linked to a lower risk for ovarian hyperstimulation syndrome. There is still a need to look for novel medicines that may be used either alone or as an adjuvant with currently existing medications due to the therapeutic failure in PCOS after taking numerous therapies.
The Food and Drug Administration (FDA), USA, approved another oral hypoglycemic medication, canagliflozin, on March 29, 2013. It is an inhibitor of the sodium-glucose cotransporter-2 (SGLT2) sodium-glucose co-transporter. The proximal tubules of the kidney express SGLT2, which promotes the reabsorption of glucose from the tubular lumen. A significant portion of the filtered glucose is eliminated by the kidney by inhibiting SGLT2. Independent of adenosine monophosphate-activated protein kinase (AMPK 1), canagliflozin boosts fatty acid oxidation and energy expenditure while decreasing adiposity, blood glucose, and the respiratory exchange ratio. Canagliflozin also reduces the expression of ATP-citrate lyase, acetyl-CoA carboxylase, and sterol response element-binding protein 1c in the liver.6
Canagliflozin also decreases body weight in nondiabetic individuals and insulin resistance in obese diabetic animals in addition to these molecular processes.7,8 According to a study by Cai et al.9 SGLT2 inhibitors should be considered as an effective drug in the treatment of patients with PCOS having insulin resistance.
This study was carried out to examine how canagliflozin affects the morphological abnormalities in the ovaries affected by PCOS. Furthermore, the combination effects of canagliflozin and metformin in PCOS were investigated.
Methods
It was a randomized experimental study conducted from March 2018 to 2019 at the Postgraduate Medical Institute, Lahore, Pakistan, after approval from the Institutional Ethics Committee.
After 2 weeks of acclimatization, a total of 40 adult female Sprague Dawley rats, weighing between 90 and 120 g and aged 7-8 weeks, were included in the study. The rats were randomly divided into six groups using the balloting technique. The normal and diseased control groups comprised eight animals each, whereas each of the other groups had six rats. To validate the induction of PCOS, two additional rats were included in the first two groups (A and B). All rats were administered letrozole (Famera, 2.5 mg, Novartis Pharmaceuticals, Pakistan) 1 mg/kg/day orally for 21 days10,11 to induce PCOS, with the exception of those in normal control group A, who received an equivalent quantity of distilled water.
On the 22nd day of the experiment, two rats, each from groups A and B, were sacrificed to confirm the induction of PCOS by histopathological analysis of the ovaries. Follicles at various developmental stages were seen in the normal control group (A). Most of the follicles in the disease control group (B) had cystic cavities filled with fluid and were atretic, which is a characteristic of ovaries with PCOS. The morphological induction of PCOS in the ovaries was verified by histological examination.
After the induction of PCOS in all rats of groups B-F till 22nd day, treatment with daily administration of medication was initiated as follows:
Group C: Canagliflozin 10 mg/kg (Invokana, 100 mg from Janssen Pharmaceutical, UAE).
Group D: Metformin 100 mg/kg (Glucophage, 500 mg from Merk Pharmaceutical, Pakistan).
Group E: High dose (HD) combination - canagliflozin 10 mg/kg/day with metformin 100 mg/kg/day.
Group F: Low dose (LD) combination - canagliflozin 05 mg/kg/day with metformin 50 mg/kg/day.12
On the 48th day of the study, rats were sacrificed after giving light anesthesia. Ovaries were removed after dissection. The weight of both ovaries was measured, and organs were preserved in 10% formalin for microscopic analysis. The tissues were processed and blocks were prepared. Horizontal sections of 3-5 μm thickness were prepared by using a microtome, and slides were made, which were stained with Hematoxylin and Eosin. Micrometry of all study groups was done. Different follicles were counted using an ocular micrometer under ×100 magnification. Follicles were categorized as primary (the presence of one cubic granulosa cell layer), secondary (the presence of two or more tall granulosa cell layers), Graafian, or big antral (antral follicle or mature follicle with a fluid-filled cavity). Atretic follicles (degenerated granulosa cells separated from the basement membrane) were also seen. Cystic fluid-filled cavities with attenuated granulosa cell layers were categorized as cystic follicles13 (Figure 1).
Statistical analysis
The Statistical Package for Social Sciences 22.0 was used to analyze the gathered data. Levene’s test was used to determine the normality of data and homogeneous variance. As a result of the data’s normal distribution, it was stated as mean SD. Analysis of variance (ANOVA) was utilized for multiple group comparisons to assess the significance of the results of quantitative data across groups. Post hoc Tukey’s test was then used to assess the mean difference between each group. Statistical significance was defined as a p-value equal to or less than 0.05.
Results
The number of primary, secondary, and cystic follicles decreased considerably (p < 0.001) by metformin and canagliflozin; however, the effects were more obvious with metformin and a high-dosage combination of metformin and canagliflozin. When compared to the disease control group, multiple comparisons using post hoc Tukey’s analysis revealed a significant reduction in the number of primary follicles in the canagliflozin (p = 0.001), metformin (D) p = 0.004, HD combination (E) p = 0.001, and LD combination (F) p = 0.002 groups. When compared to the disease control group, the number of secondary follicles in the high-dosage combination group (E) was considerably reduced (p = 0.001). Nevertheless, the findings revealed that only metformin (group D) substantially increased the frequency of Graafian follicles (p-value of 0.01) as compared to the disease control group. Metformin also significantly reduced the quantity of atretic follicles when combined with low and HD (p = 0.01, 0.002, and 0.01), respectively. The number of corpus luteum increased considerably in the groups of canagliflozin (C), metformin (D), and HD combination (E) (p = 0.01, 0.001, and 0.001), respectively. Cystic follicles, a characteristic of polycystic illness, were significantly reduced in all treatment groups by p = 0.003, p = 0.001, p = 0.001, and p = 0.001 in canagliflozin (C), metformin (D), high dosage combination (E), and LD combination group (F), respectively, as compared to the disease control group. When compared to the canagliflozin-treated group, the high-dosage combination group showed a significantly decreased number of cystic follicles (p = 0.01) (Table 1).
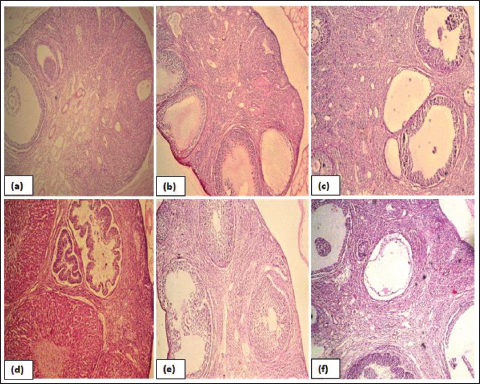
Figure 1. Photomicrographs of the ovarian section, observed under 10×. (a) Normal control group showing secondary and Graafian follicles, (b) disease control group, note the presence of multiple subcapsular fluid-filled cysts of different sizes, (c) canagliflozin-treated group shows follicles in all stages of development with few cystic follicles, (d) metformin-treated rats with corpus luteum at different stages of development, (e) HD combination treated rats with follicles at different stages of development, and (f) LD combination treated rats with the presence of cystic follicle and mature follicle.
Discussion
The effects of PCOS on a woman’s health and well-being are severe. Despite its high prevalence, PCOS and the associated morbidities are probably underdiagnosed.14 In the current study, letrozole was used to induce PCOS through an increase in androgen levels, thus producing cysts in ovaries that are histologically comparable to those found in humans with PCOS.15,16
In the current study, only histopathology of the cystic disease of the ovaries was focused upon instead of metabolic derangements of PCOS.16 It is documented in the literature that androgen accelerates the growth of follicles and their transition from primordial to primary to secondary follicles. This study found that the disease control group (B) had a substantial rise in primary (6.5 ± 1.87), secondary (3.66 ± 1.21), and large cystic follicles (2.53 ± 1.04) as compared to the normal control group because of the relative deficiencies in follicle-stimulating hormone and progesterone levels. The number of atretic follicles was increased in the disease group (B), and as reported in the literature, larger follicles showed a greater degree of atresia.17 Assumption can be made that letrozole increases the number of antral follicles, but most of them undergo atresia before ovulation.
The second objective of the current study was to examine how metformin and canagliflozin impacted the histological aberrations in rat ovaries with PCOS. Canagliflozin has a similar mechanism of action to metformin, a drug that is FDA-approved for treating PCOS. The results of the present study show that canagliflozin enhances the effects of metformin when taken at HD; however, the impact was less pronounced than it was with metformin in terms of ovarian histology.
Table 1. Effect of canagliflozin and metformin on number of different follicles in ovaries of letrozole induced PCOS rat model.
| Follicles | A Normal control |
B Disease control |
C Canagliflozin |
D Metformin |
E HD |
F LD |
ANOVA Sig. |
|---|---|---|---|---|---|---|---|
| Primary follicles | 3.60 ± 0.81* | 6.5 ± 1.87 | 3.13 ± 0.76* | 3.66 ± 1.25* | 2.83 ± 0.75* | 3.50 ± 0.75* | <0.001 |
| Secondary follicles | 1.16 ± 0.75* | 3.66 ± 1.21 | 2.83 ± 0.074 | 1.66 ± 0.51* | 1.33 ± 0.84* | 2.66 ± 0.81 | <0.011 |
| Graafian follicles | 1.10 ± 0.75 | 0.16 ± 0.45 | 1.00 ± 0.89 | 1.53 ± 0.51* | 0.66 ± 0.51 | 0.50 ± 0.54 | 0.012 |
| Atretic follicles | 2.16 ± 0.75* | 5.66 ± 2.87 | 3.66 ± 0.81 | 2.83 ± 0.75* | 2.16 ± 1.16* | 2.67 ± 0.51* | 0.001 |
| Corpus luteum | 1.67 ± 0.81 | 0.33 ± 0.51 | 3.50 ± 1.37* | 3.83 ± 0.76*b | 4.00 ± 1.9*b | 2.33 ± 1.86 | <0.001 |
| Cystic follicles | 0.00 ± 0.00* | 2.53 ± 1.04 | 1.16 ± 0.40* | 0.66 ± 0.51* | 0.33 ± 0.5* a | 0.50 ± 0.54* | <0.001 |
*Significant of ≤0.05 indicated by asterisk (*) as compared to disease control group.
aPost hoc Tukey test shows that the HD group has a significant reduction in cystic follicles as compared to canagliflozin and metformin alone, whereas bmetformin and HD combination groups showed an increased number of mature corpus luteum as compared to the normal control group.
Cystic follicles are a defining feature of PCOS, and all therapy groups showed a reduction in their number when compared to the disease control group. Also, it was found that group E, which received an HD combination of both drugs, had the fewest cystic follicles (p = 0.001), indicating that the combination of both treatments had a favorable impact on PCOS’s histopathological changes.
In all treatment groups, fewer primary and secondary follicles were seen, but there was no distinction among the groups. Only the metformin therapy group (group D) showed a significantly greater number of Graafian follicles (1,530.51) when we evaluated the number of Graafian follicles that decreased following letrozole treatment in the disease control group as compared to the normal control group.
After receiving metformin medication, the degree of atresia in the ovaries was significantly (p = 0.01) decreased. As compared to the disease control group, canagliflozin did not significantly reduce the number of atretic follicles (p = 0.16), but when combined with metformin, the results were significant (group E; p = 0.002 and group F; p = 0.01), which may be due to the positive effects of metformin on its own as seen in a prior study by Mahamed et al.18 who reported that metformin reduces the number of primary and secondary follicles, lessens the quantity of interstitial cells and degenerating follicles, leads to the proliferation of the theca interna and interstitial cells, as evidenced by the expression of Ki-67 and vascular endothelial growth factor-A. In addition, metformin also improved angiogenesis.18
This study examined the effects of canagliflozin and metformin both separately and in combination on PCOS-related ovarian dysregulations; however, it did not examine any parameters to determine a potential mechanism. PCOS and insulin resistance with hyperandrogenism have complicated relationships. Due to an increase in androgen release from the adrenal cortex and ovaries, insulin resistance can directly increase the risk of PCOS in obese people. Insulin can bind to the insulin receptor and Insulin like Growth Factor-1 (IGF-1) on the adrenal cortex and promote theca cell proliferation, which in turn causes follicular cysts in the ovaries to grow more quickly.19
Another mechanism seen in PCOS is a reduction in AMPK expression in many ovarian cells, including the oocyte, cumulus cells, granulosa cells, corpus luteal, and theca cells. Metformin has been found to boost AMPK expression in theca cells. As a result, metformin treatment for PCOS patients may help to improve ovulatory dysfunction because of the activation of AMPK in the ovary itself.20 Canagliflozin also increases AMPK by inhibiting mitochondrial function and, in turn, increasing Adenosine monophosphate (AMP) (AMP) in cells,21 which can be a possible mechanism for the correction of ovarian dysfunction. In a 12-week, open-label, randomized trial comparing empagliflozin to metformin in obese PCOS-afflicted women, empagliflozin therapy resulted in a substantial improvement in anthropometric measurements and body composition, but no changes were seen in metabolic measurements.22 There is still a need to further evaluate the effects of canagliflozin on the biochemical and pathophysiological mechanisms involved in the induction and progression of PCOS.
Conclusion
Based on the results of this study, it is concluded that a combination of metformin with canagliflozin is more effective than either drug alone in ameliorating the histological changes in ovaries of an experimental model of PCOS.
Limitations of the Study
To determine the effects of the canagliflozin on the rat model of PCOS, limited histological parameters were analyzed. This study did not include the histochemical or biochemical alterations or the underlying molecular mechanism of amelioration in the histological changes of PCOS.
Acknowledgement
The authors are pleased to acknowledge the guidance and suggestions of Dr. Munnaza Hassan, Associate Professor of the Pathology Department of Lahore General Hospital Lahore, Pakistan, which made it possible to carry out all histological work. Special thanks to Dr. Amir Siddique for the statistical analysis of data, the laboratory staff of the Pharmacology Department, and the workers of the animal house of Post Graduate Medical Institute, Lahore, Pakistan, who provided their full support during this research work.
List of Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| ANOVA | Analysis of variance |
| HD | High dose combination group |
| LD | Low dose combination group |
| PCOS | Polycystic ovarian syndrome |
| SGLT2 | Sodium glucose co-transporter-2 |
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose. This study is extracted from the M.Phil research work of the Principal author while she was a resident of Pathology at Postgraduate Medical Institute Lahore, Pakistan.
Ethical approval
Ethical approval for the study was obtained from the Institutional Review Board of Post Graduate Medical Institute (PGMI) Lahore, Pakistan, vide Letter No. (00-28-S-2017) dated 11-11-2017.
Authors’ contributions
AZ, RM, SC: Conception of work, acquisition and analysis of data
SM: Drafting the manuscript and acquisition of data
QA and HMIA: Revising the manuscript critically for important intellectual content
ALL AUTHORS: Approval of the final version of the manuscript to be published
Authors’ Details
Akfish Zaheer1, Rabab Miraj2, Sidra Mushtaq1, Qurat-tul-Ain3, Hafiz Muhammad Imran Aziz4, Sadia Chiragh5
- Assistant Professor, Department of Pharmacology, Independent Medical College, Faisalabad, Pakistan
- Assistant Professor, Department of Pharmacology, Sialkot Medical College, Sialkot, Pakistan
- Assistant Professor, Department of Pharmacology, Pak Red Crescent Medical and Dental College, Lahore, Pakistan
- Associate Professor, Department of Pharmacology, ABWA Medical College, Faisalabad, Pakistan
- Head, Department of Pharmacology, Al-Aleem Medical and Dental College, Postgraduate Medical Institute, Lahore, Pakistan
References
- Joham AE, Norman RJ, Stener-Victorin E, Legro RS, Franks S, Moran LJ, et al. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022 Sep;10(9):668–80. https://doi.org/10.1016/S2213-8587(22)00163-2
- Aversa A, La Vignera S, Rago R, Gambineri A, Nappi RE, Calogero AE, et al. Fundamental concepts and novel aspects of polycystic ovarian syndrome: expert consensus resolutions. Front Endocrinol (Lausanne). 2020 Aug;11:516–32. https://doi.org/10.3389/fendo.2020.00516
- Helvaci N, Yildiz BO. Current and emerging drug treatment strategies for polycystic ovary syndrome. Expert Opin Drug Deliv. 2022;24(1):105–120. https://doi.org/10.1080/14656566.2022.2108702
- Krysiak R, Szkróbka W, Bednarska-Czerwińska A, Okopień B. Plasma gonadotropin levels in metformin-treated men with prediabetes: a non-randomized, uncontrolled pilot study. Fundam Clin Pharmacol. 2021 Apr;35(2):466–72. https://doi.org/10.1111/fcp.12600
- Kojok D, Ghazeeri G, Awwad JT. Role of insulin-sensitizing drugs in management of polycystic ovarian syndrome. Cur Emerg Concepts. 2022;233–53. https://doi.org/10.1007/978-3-030-92589-5_12
- Koike Y, Shirabe SI, Maeda H, Yoshimoto A, Arai K, Kumakura A, et al. Effect of canagliflozin on the overall clinical state including insulin resistance in Japanese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019 Mar;149:140–6. https://doi.org/10.1016/j.diabres.2019.01.029
- Yoshino K, Hosooka T, Shinohara M, Aoki C, Hosokawa Y, Imamori M, et al. Canagliflozin ameliorates hepatic fat deposition in obese diabetic mice: role of prostaglandin E2. Biochem Biophys Res Commun. 2021 Jun;557:62–8. https://doi.org/10.1016/j.bbrc.2021.04.012
- Wei D, Liao L, Wang H, Zhang W, Wang T, Xu Z. Canagliflozin ameliorates obesity by improving mitochondrial function and fatty acid oxidation via PPARα in vivo and in vitro. Life Sci. 2020 Apr;247:117414. https://doi.org/10.1016/j.lfs.2020.117414
- Cai M, Shao X, Xing F, Zhang Y, Gao X, Zeng Q, et al. Efficacy of canagliflozin versus metformin in women with polycystic ovary syndrome: a randomized, open-label, noninferiority trial. Diabetes Obes Metab. 2022 Feb;24(2):312–20. https://doi.org/10.1111/dom.14583
- Hong Y, Yin Y, Tan Y, Hong K, Zhou H. The flavanone, naringenin, modifies antioxidant and steroidogenic enzyme activity in a rat model of letrozole-induced polycystic ovary syndrome. Med Sci Monit. 2019 Jan;25:395–401. https://doi.org/10.12659/MSM.912341
- Kafali H, Iriadam M, Ozardali I, Demir N. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 2004;35(2):103–8. https://doi.org/10.1016/j.arcmed.2003.10.005
- Marie MA, Arafa NM, Alazimi SA. Effect of canagliflozin or metformin on metabolic disorders in obese diabetic rats. Afr J Pharm Pharmacol. 2015;9(46):1071–9. https://doi.org/10.5897/AJPP2015.4455
- Groppetti D, Pecile A, Frattini S, Pagnacco G, Arrighi S. Histological feature of ovarian structures throughout the reproductive cycle in Alpine goats. Maced Vet Rev. 2018;42(1):23–34. https://doi.org/10.2478/macvetrev-2018-0027
- Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018 Sep;33(9):1602–18.
- Stener-Victorin E, Padmanabhan V, Walters KA, Campbell RE, Benrick A, Giacobini P, et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocr Rev. 2020 Jul;41(4):1–39. https://doi.org/10.1210/endrev/bnaa010
- Franks S, Hardy K. Androgen action in the ovary. Front Endocrinol (Lausanne). 2018 Aug;9:452. https://doi.org/10.3389/fendo.2018.00452
- Wang MX, Yin Q, Xu X. A rat model of polycystic ovary syndrome with insulin resistance induced by letrozole combined with high fat diet. Med Sci Monit. 2020 May;26:e922136–1. https://doi.org/10.12659/MSM.922136
- Mahamed RR, Maganhin CC, Sasso GR, de Jesus Simões M, Baracat MC, Baracat EC, et al. Metformin improves ovarian follicle dynamics by reducing theca cell proliferation and CYP-17 expression in an androgenized rat model. J Ovarian Res. 2018 Mar;11(1):18. https://doi.org/10.1186/s13048-018-0392-1
- Zeng X, Xie YJ, Liu YT, Long SL, Mo ZC. Polycystic ovarian syndrome: correlation between hyperandrogenism, insulin resistance and obesity. Clin Chim Acta. 2020 Mar;502:214–21. https://doi.org/10.1016/j.cca.2019.11.003
- Wen KC, Sung PL, Wu AT, Chou PC, Lin JH, Huang CF, et al. Neoadjuvant metformin added to conventional chemotherapy synergizes anti-proliferative effects in ovarian cancer. J Ovarian Res. 2020 Aug;13(1):95. https://doi.org/10.1186/s13048-020-00703-x
- Shoda K, Tsuji S, Nakamura S, Egashira Y, Enomoto Y, Nakayama N, et al. Canagliflozin inhibits glioblastoma growth and proliferation by activating AMPK. Cell Mol Neurobiol. 2022;18:1–4. https://doi.org/10.1007/s10571-022-01221-8
- Javed Z, Papageorgiou M, Deshmukh H, Rigby AS, Qamar U, Abbas J, et al. Effects of empagliflozin on metabolic parameters in polycystic ovary syndrome: a randomized controlled study. Clin Endocrinol (Oxf). 2019 Jun;90(6):805–13. https://doi.org/10.1111/cen.13968
