Original Article
VOLUME: 37 | ISSUE: 4 | Dec 25, 2021 | PAGE: (220 - 226) | DOI: 10.51441/BioMedica/5-296
Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection
Authors: Rabia Nafees , Haroon Latif Khan , Yousaf Latif Khan , Aisha Awais , Miss Anum Farooqi , Rameen Nisar
Article Info
Authors
Rabia Nafees
Lahore General Hospital / Hameed Lateef Hospital, Lahore, Pakistan
Haroon Latif Khan
Chief Executive, Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan
Yousaf Latif Khan
Consultant, Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan
Aisha Awais
Consultant, Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan
Miss Anum Farooqi
Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan
Rameen Nisar
Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan
Publication History
Received: July 07, 2021
Revised: September 10, 2021
Accepted: November 21, 2021
Published: December 25, 2021
Abstract
Background & Objective:
Assisted reproductive technique is an evolving field with many recent advances. The success rate is low in developing countries where financial concerns prevail predominantly. This study was designed for the first time in any hospital in Pakistan to determine the role of Endometrial Receptivity Array (ERA) in patients with previous implantation failure to improve pregnancy outcome and to enhance the success rate of in-vitro fertilization & intracytoplasmic sperm injection (IVF/ICSI).
Methods:
This study was carried out at the Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan. A total of 16 patients were recruited after taking written informed consent. Only those patients were selected who had previous one or more implantation failures in IVF/ICSI cycles and had at least 2 or more good quality frozen embryos. RNA was obtained from the endometrial sample to check ERA through 238 genes expressed using RNA sequencing. Beta HcG level and scans were performed to confirm the clinical pregnancy.
Results:
All enrolled patients had an ERA test and their embryos transferred according to personalized window of implantation (WOI). A total of 5(31.3 %) patients were stimulated with a long protocol while 11 (68.7%) underwent a short protocol. WOI was receptive in 12 (75%) patients, pre-receptive in 3 (18.2%) and post-receptive in 1 (6.2%), and most of patients showed receptivity at P5 (109-145 hours). Twelve patients (75%) had clinical pregnancy evident by positive beta HCG after embryo transfer. A significant association was found between WOI and receptivity (P<0.05).
Conclusion:
The results of ERA in our study seem promising especially in our patients with previous one or more implantation failures. Although we have limited number of patients keeping in mind its financial constraints especially in developing countries, still ERA is considered a way of hope especially for those patients who have previous implantation failures.
Keywords: Endometrial Receptivity Array (ERA), Window of Implantation (WOI), Trans-vaginal Scan (TVS), Frozen Embryo Transfer (FET), In Vitro Fertilization (IVF), Intracytoplasmic sperm injection (ICSI)
Pubmed Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar. Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection. BioMedica. 2021; 25 (December 2021): 220-226. doi:10.51441/BioMedica/5-296
Web Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar. Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection. https://biomedicapk.com/articles/online_first/296 [Access: July 27, 2024]. doi:10.51441/BioMedica/5-296
AMA (American Medical Association) Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar. Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection. BioMedica. 2021; 25 (December 2021): 220-226. doi:10.51441/BioMedica/5-296
Vancouver/ICMJE Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar. Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection. BioMedica. (2021), [cited July 27, 2024]; 25 (December 2021): 220-226. doi:10.51441/BioMedica/5-296
Harvard Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar (2021) Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection. BioMedica, 25 (December 2021): 220-226. doi:10.51441/BioMedica/5-296
Chicago Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar. "Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection." 25 (2021), 220-226. doi:10.51441/BioMedica/5-296
MLA (The Modern Language Association) Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar. "Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection." 25.December 2021 (2021), 220-226. Print. doi:10.51441/BioMedica/5-296
APA (American Psychological Association) Style
Rabia Nafees, Haroon Latif Khan, Yousaf Latif Khan, Aisha Awais, Miss Anum Farooqi, Rameen Nisar (2021) Role of Endometrial Receptivity Array for Implantation failure in In-Vitro Fertilization & Intracytoplasmic Sperm Injection. , 25 (December 2021), 220-226. doi:10.51441/BioMedica/5-296
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(4):220-226
ORIGINAL ARTICLE
Role of endometrial receptivity array for implantation failure in in-vitro fertilization & intracytoplasmic sperm injection
Rabia Nafees1*, Haroon Latif Khan2, Yousaf Latif Khan3, Aisha Awais4, Anum Farooqi5, Rameen Nisar6
Received: 07 July 2021 Revised date: 10 August 2021 Accepted: 21 November 2021
Correspondence to: Rabia Nafees
*Assistant Professor, Department of Obstetrics & Gynecology, Rashid Latif Medical College, Lahore, Pakistan.
Email: rabia_nafees@hotmail.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Assisted reproductive technique is an evolving field with many recent advances. The success rate is low in developing countries where financial concerns prevail predominantly. This prospective study was designed for the first time in any hospital in Pakistan to determine the role of Endometrial Receptivity Array (ERA) in patients with previous implantation failure to improve pregnancy outcome and to enhance the success rate of in vitro fertilization & intracytoplasmic sperm injection (IVF/ICSI).
Methods:
This study was carried out at the Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan from December, 2019 to October, 2020. A total of 16 patients were recruited after taking written informed consent. Only those patients were selected who had previous one or more implantation failures in IVF/ICSI cycles and had at least two or more good quality frozen embryos. RNA was obtained from the endometrial sample to check ERA through 238 genes expressed using RNA sequencing. Beta HCG level and scans were performed to confirm the clinical pregnancy.
Results:
All enrolled patients had an ERA test and their embryos transferred according to personalized window of implantation (WOI). A total of 5 (31.3%) patients were stimulated with a long protocol while 11 (68.7%) underwent a short protocol. WOI was receptive in 12 (75%) patients, pre-receptive in 3 (18.2%) and post-receptive in 1 (6.2%), and most of patients showed receptivity at P5 (109-145 hours). Twelve patients (75%) had clinical pregnancy evident by positive beta human chorionic gonadotrophin (HCG) after embryo transfer. A significant association was found between WOI and receptivity (P < 0.05).
Conclusion:
The results of ERA in our study seem promising, especially in patients with previous one or more implantation failures. Although we have limited number of patients keeping in mind its financial constraints, especially in the developing countries, still ERA is considered a way of hope, especially for those patients who have previous implantation failures.
Keywords:
Endometrial Receptivity Array (ERA), window of implantation (WOI), trans-vaginal scan (TVS), frozen embryo transfer (FET), in vitro Fertilization (IVF), intracytoplasmic sperm injection (ICSI).
Introduction
Even though Pakistan is among the most crowded nations globally and has a populace development pace of around 2%, it likewise has a high infertility ratio (21.9%); 3.5% primary, and 18.4% secondary.1 Furthermore, psychological impact associated with infertility and its treatment is still challenging.2 Since the birth of first in vitro fertilization (IVF) baby, there were many hopes about the success of IVF, but the success rate of IVF and intracytoplasmic sperm injection (ICSI) is still not up to the desired level.3 It needs to be improved further, notably in the developing countries, where finances are an impelling problem; patients pay for the treatment out of their pocket; hence, they avail a limited number of treatment attempts.4
Despite achieving good quality embryos, success rate of IVF & ICSI stagnates at a minimum standard. The major contributing factor of low success in such patients is implantation failure.5 Successful IVF is a synchronized and timely transfer of a good quality embryo to the welcoming endometrium.6 The tight time frame during which the endometrium is ready to receive a healthy embryo is called the “window of implantation” (WOI).7
In normal IVF & ICSI cycle, it is assumed that every patient has a fixed WOI, transfer is usually timed around days 3 or 5.8 However, few patients’ windows are slightly away from mainstream and hence suffer from implantation failure. Hence, problem is that if good quality embryos fail to implant repeatedly, how endometrial receptivity (ER) can be checked to ascertain high chances of displaced WOI in such patients? The answer to the problem could be Endometrial Receptivity Array (ERA). The basis for ERA is that each woman has a unique WOI, and by knowing this factor, the chances of successful pregnancy can be enhanced. This is called “personalized embryo transfer” (PET) (Figure 1).
ERA is an effort to reveal the personalized WOI, particularly, in patients with previous implantation failure. Recurrent implantation failure is defined as “failure to achieve pregnancy after embryo transfer”.10 A total of 73% failed cycles occur due to failed embryo implantation either due to aneuploid embryo or implantation failure. Three in every 10 women have a displaced WOI failure.9
Another thought is that implantation failure is not pathology, but it is the inability to develop accurate time- based harmony between the developing embryo and the endometrium.11 ERA is a technique to see genomic expression of endometrium to time embryo transfer. Data derived suggests that embryo transfer timing is decided by expression of genes from endometrium. Total RNA is obtained from endometrial biopsy and used for genomic expression to check receptivity.12 ERA evaluates 238 genes expressed during WOI using RNA sequencing.13 Following analysis, endometrium is classified as receptive or non- receptive (NR). The NR endometrium is further classified as pre or post receptive, meaning that the endometrium has not reached the receptive phase yet or has already passed it, respectively.14 Recurrent implantation failures may be due to a previously deserted WOI. These are the patients who are candidate for PET decided by ERA.14
Methods
It was a prospective study on couples coming for the IVF/ ICSI at the Lahore Institute of Fertility & Endocrinology, Lahore-Pakistan from December, 2019 to October, 2020. The institutional ethical review committee approved this study. We selected 16 patients for ERA, and written informed consent was obtained. We selected only those patients with previous one or more implantation failures in IVF/ICSI cycles and have at least two or more good quality frozen embryos. All cases of hydrosalpinx, submucous fibroid or with previous difficult ET were excluded.
For ERA, there were two cycles. First was Biopsy Cycle, which was initiated by prescribing tablet Progynova 2 mg three times a day from first day of the cycle followed by trans-vaginal scan on cycle day-8 for endometrial thickness that should be >6 mm. In addition, serum progesterone (P4) levels were checked and ensured that they were <1 ng. After that, P4 pessary was inserted from CD-9. Endometrial biopsy was performed on P + 5 or 120th hour after starting P4. Biopsy results usually took 2-3 weeks. Endometrial sample was sent to Igenomix lab (Dubai) with all precautionary measures. RNA was obtained from the endometrium sample, and it was used for genomic expression to check its receptivity. The result was either receptive or NR endometrium. A receptive endometrium showed that the WOI was located on taking the sample. The recommendation was to proceed with embryo transfer under the same condition as for biopsy. A NR result showed a displaced WOI. In that case, with the ERA computational predictor, we estimated WOI by another biopsy. The NR endometrium was further classified as pre or post receptive. A biopsy cycle was repeated according to displaced WOI until we got the receptive endometrium.
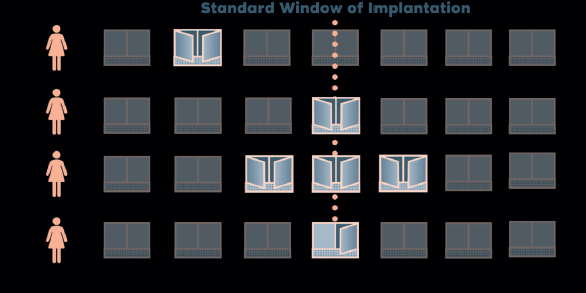
Figure 1. Window of implantation (courtesy IGENOMICS).9
The second cycle is transfer cycle, similar to biopsy cycle for embryo transfer as calculated by ERA WOI.
Statistical analysis
The data were analyzed using SPSS-25.0. For categorical variables descriptive analysis and for numerical variables mean and standard deviation was calculated. Pearson Chi-Square test was used to find the association between different variables with the 0.05% or 5% level of significance.
Results
The mean age of the patients was 32.25 ± 4.38 and body mass index (BMI) was 25.31 ± 3.51. The mean baseline hormonal parameters of follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, estradiol (E2), and anti- Mullerian hormone (AMH) was 6.02 ± 1.69, 6.34 ± 4.78, 16.28 ± 10.77, 45.61 ± 30.69, and 4.76 ± 3.54, respectively. Similarly, the mean thyroid stimulating hormone (TSH) level was 2.62 ± 2.36, triiodothyronine (T3) was 8.89 ± 2.90, and thyroxin (T4) was 15.10 ± 30.52. In these selective patients, Rubella antibodies IgG mean was 88.80 ± 93.68, and IgM was 0.47 ± 0.26, therefore considered negative.
Primary and secondary infertility were reported by 9 (56.3%) and 7 (43.7%) patients, respectively. Most women had regular menstrual cycle 14 (87.5%), while 2 (12.5%) had irregular cycle.
Regarding etiology of infertility, 4 (25.0%) had tubal factor, 3 (18.3%) had unexplained infertility, 5 (31.3%) had male factor, and 4 (25%) had both male and female factor
Out of 16 patients who were stimulated, 5 (31.3%) underwent long protocol and 11 (68.7%) had a short protocol. Furthermore, regarding antral follicle count (AFC), 3 (18.7%) had 8-15, and 13 (81.3%) had more than 15. The mean value of serum E2 & P4 levels and endometrial thickness on the day of decision were 3,468.36 ± 113.9 iu, 5.74 ± 2.39, 10.09 ± 1.43 mm, respectively (Table 1).
Mean of total oocyte (both mature and immature) count was 17.50 ± 6.67, 14.88 ± 5.59, and 2.63 ± 2.60, respectively. Single and double embryo were transferred in 7 (43.75%), more than two embryos were transferred in 2 (12.5%) patients. Out of 16, 2 (12.5%) were early blastocysts, 7 (43.8%) were expanded blastocysts, 5 (31.3%) were hatched, and 2 (12.4%) were morula (Table 3).
WOI was receptive in 12 (75%) patients, pre-receptive in 3 (18.2%), and post-receptive in 1 (6.2%), and most of patients showed receptivity at P5 (109-145 hours) (Table 2).
The significant association was found between WOI and receptivity (P < 0.05). Beta HCG was positive in 12 (75%) patients, and out of these, 8 (67%) patients had fetal cardiac activity (FCA) (Table 4).
Discussion
ART in Pakistan is evolving but at a very slow pace and with limited available data. ERA is an attempt to introduce new techniques in IVF/ICSI to enhance its success rate. It is a ray of hope particularly for those patients who have previous implantation failure in Pakistan as this test is introduced for the first time. Life Center Lahore is the first and the only center in Pakistan carrying out this test. Finances and resources both are the limiting factor for any new advancement particularly in developing countries. Hence, ERA in Pakistan with only 16 patients is justifiable as it opens the door for future innovation in IVF with limited resources.
The success of human implantation is a complex process depending upon a good quality embryo and endometrium ready to receive this embryo and a synchronous harmony amongst them.15,16 Apart from the good quality embryo; ER is a big challenge now a days with limited available tests. Furthermore, the functional tests for endometrium are less accurate and have low predictive value. Microarray innovation (ERA) has permitted recognizable proof of the transcriptomic mark of the WOI. Subsequently, it leads to the improvement of an ER measure (time) for the finding of adjusted ER.17 Therefore, genomic expression of endometrium is an attempt to enhance the implantation rate in IVF & ICSI.13,18
The personalized way of embryo transfer enhances the success rate of ART, and it also enhances the clinical pregnancy rate. A total of 75% of our patient’s ERA results showed that endometrium was found to be receptive which is comparable with the study of Patel et al.7 ERA helps finding out a personalized WOI for majority of these patients with previous implantation failure. In addition, the results of positive beta HCG in our 12 patients, as a marker of receptivity, are comparable to the study of Simon et al.12 and Mahajan et al.16
ERA test is accurate and sensitive in identifying genetic expressions of the endometrium to pinpoint embryo transfer timing.19,20 PET guided by ERA significantly improves pregnancy rates in patients with unexplained repeated implantation failure.7 Although we have limited available data but results of ERA are promising. This test is still in an evolution phase and the authors are looking forwards to establish a local lab in Pakistan so that the financial issues and time delays can be overcome to get the maximum benefit for the public in Pakistan.
Table 1. Demographic parameters of ERA patients.
| Parameters | Mean ± SD |
|---|---|
| Mean Age (years) | 32.25 ± 4.38 |
| Mean BMI (kg/m2) | 25.31 ± 3.51 |
| Infertility diagnosis | |
| Mean duration of infertility (years) | 6.25 ± 4.15 |
| Type of infertility n (%) | |
| Primary | 9 (56.3) |
| Secondary | 7 (43.7) |
| No. of attempts | 2.25 ± 1.12 |
| Menstrual cycle n (%) | |
| Regular | 14 (87.5) |
| Irregular | 2 (12.5) |
| Baseline hormonal parameters (mean ± SD) | |
| FSH mIU/ml | 6.02 ± 1.69 |
| LH | 6.34 ± 4.78 |
| Prolactin ng/ml | 16.28 ± 10.77 |
| E2 pg/ml | 45.61 ± 30.69 |
| AMH ng/ml | 4.76 ± 3.54 |
| TSH µIU/ml | 2.62 ± 2.36 |
| Infertility etiology n (%) | |
| Tubal | 4 (25.0) |
| Unexplained infertility factor | 3 (18.3) |
| Male factor | 5 (31.3) |
| Both Male & Female factor | 4 (25.0) |
| Stimulation parameters n (%) | |
| Protocol | |
| Long | 5 (31.3) |
| Short | 11 (68.7) |
| Ultrasonography parameters n (%) AFC | |
| <8 | - |
| 8-15 | 3 (18.7) |
| >15 | 13 (81.3) |
| Serum endocrine levels on decision day (mean ± SD) | |
| E2 | 3,468.36 ± 113.9 |
| P4 | 5.74 ± 2.39 |
| Endometrium thickness | 10.09 ± 1.43 |
Data are presented as mean and standard deviation for continuous variables and n (%) for categorical variable.
Table 2. Window of implantation of ERA patients.
| Age | No. of attempts | WOI | Hours | Receptivity | No. of ET | Clinical pregnancy | FCA |
|---|---|---|---|---|---|---|---|
| 34 | 3.00 | P + 5 | 110.00 | R | 2.00 | Positive | Negative |
| 32 | 3.00 | P + 5 | 115.00 | R | 2.00 | Positive | Positive |
| 28 | 1.00 | P + 5 | 120.00 | R | 2.00 | Positive | Positive |
| 30 | 1.00 | P + 5 | 120.00 | R | 1.00 | Positive | Negative |
| 29 | 3.00 | P + 5 | 109.00 | R | 1.00 | Positive | positive |
| 39 | 2.00 | P + 5 | 121.00 | R | 1.00 | Positive | Negative |
| 30 | 1.00 | P + 5 | 120.00 | R | 1.00 | Negative | Negative |
| 26 | 1.00 | P + 5 | 145.00 | pre | 3.00 | Positive | Positive |
| 39 | 4.00 | P + 5 | 108.00 | R | 2.00 | Positive | Positive |
| 37 | 3.00 | P + 5 | 145.00 | pre | 1.00 | Positive | Negative |
| 24 | 1.00 | P + 4 | 96.00 | post | 3.00 | Negative | Negative |
| 32 | 1.00 | P + 5 | 110.00 | R | 2.00 | Negative | Negative |
| 36 | 2.00 | P + 5 | 108.00 | R | 1.00 | Positive | Positive |
| 34 | 3.00 | P + 4 | 108.00 | pre | 2.00 | Positive | Positive |
| 31 | 4.00 | P + 5 | 125.00 | R | 1.00 | Positive | Positive |
| 35 | 3.00 | P + 5 | 145.00 | R | 2.00 | Negative | Negative |
Table 3. Outcome parameters of ART treatment of ERA patients.
| Parameters | Mean ± SD |
|---|---|
| Total oocytes | 17.50 ± 6.67 |
| Mature | 14.88 ± 5.59 |
| Immature | 2.63 ± 2.60 |
| No of embryos [n (%)] | |
| 1 | 7 (43.7) |
| 2 | 7 (43.7) |
| 3 | 2 (12.5) |
| Embryo stage [n (%)] | |
| Early blastocysts | 2 (12.5) |
| Expanded blastocysts | 7 (43.8) |
| Hatched blastocysts | 5 (31.3) |
| Morula | 2 (12.4) |
Conclusion
The ERA results in this study seem promising, especially in patients with previous one or more implantation failures. Although the study recruited limited number of patients keeping in mind the financial constraints, especially in developing countries like Pakistan, ERA may still be considered a way of hope, especially for patients with previous implantation failures.
Limitations of the study
The major limiting factor for the study is the small number of patients for ERA because of high procedure cost. ERA is an expensive test and labs are not currently available in Pakistan so we had to send the samples abroad for results which led to delays.
Table 4. Pregnancy outcomes in ERA patients.
| Variables | n (%) | |
|---|---|---|
| No. of failed attempts | 0 | 1 (6.3) |
| 1 | 2 (12.5) | |
| 2 | 8 (50.0) | |
| 3 | 4 (25.0) | |
| 4 | 1 (6.3) | |
| WOI | Receptive | 12 (75.0) |
| Pre-receptive | 3 (18.2) | |
| Post-receptive | 1 (6.2) | |
| Hours of WOI | P4 (96-108) | 2 (12.5) |
| P5 (109-145) | 14 (87.5) | |
| Clinical pregnancy | Beta HCG positive | 12 (75.0) |
| Beta HCG negative | 4 (25.0) | |
| FCA | Present | 8 (67.0) |
| Absent | 4 (33.0) |
Acknowledgement
The authors are thankful to Dr. Rashid Latif Khan for motivating and guiding for the research. A special thanks to Dr. Shahzad Bhatti & the whole team of Lahore Institute of Fertility & Endocrinology particularly Dr. Nighat Mehmood & Miss Shezae for their help and support.
List of Abbreviations
| E2 | Estradiol |
| ERA | Endometrial Receptivity Array |
| ICSI | Intracytoplasmic sperm injection |
| IVF | In vitro fertilization |
| P4 | Progesterone |
| PET | personalized embryo transfer WOI Window of implantation |
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose.
Ethical approval
Ethical approval was granted by the Ethics Committee, Lahore Institute of Fertility & Endocrinology, Lahore, Pakistan vide letter No IRB/2019/012 dated 06-10-2019.
Authors’ contribution
RN: Study design, acquisition and analysis of data
HLK: Conception of study and drafting of manuscript
YLK: Study design and drafting of manuscript
AA, AF: Data collection and drafting of manuscript
RN: Acquisition and analysis of data
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ details
Rabia Nafees1, Haroon Latif Khan2, Yousaf Latif Khan1,3, Aisha Awais4, Anum Farooqi5, Rameen Nisar6
- Assistant Professor, Department of Obstetrics & Gynecology, Rashid Latif Medical College, Lahore, Pakistan
- Chief Executive, Lahore Institute of Fertility & Endocrinology, Lahore, Pakistan
- Consultant, Lahore Institute of Fertility & Endocrinology, Lahore, Pakistan
- IVF Consultant, Lahore Institute of Fertility & Endocrinology, Lahore, Pakistan
- Embryologist, Lahore Institute of Fertility & Endocrinology, Lahore, Pakistan
- Biostatistician & Research Assistant, Lahore Institute of Fertility & Endocrinology, Lahore, Pakistan
References
- Shaheen R, Subhan F, SultanS, Subhan K, Tahir F. Prevalence of infertility in a cross section of Pakistani population. Department of obstetrics and gynecology, Federal government services hospital, Islamabad, Pakistan and Department of reproductive Physiology/health, Public health laboratories division, National Institute of Health, Islamabad, Pakistan (FS, SS, KS, FT). Pak J Zool. 2010;42(4):389–93.
- Qadir F, Khalid A, Medhin G. Social support, marital adjustment, and psychological distress among women with primary infertility in Pakistan. Women Health. 2015;55(4):432–46. https://doi.org/10.1080/03630242.2015.1022687
- Heijnen EMEW, Macklon NS, Fauser BCJM. What is the most relevant standard of success in assisted reproduction? The next step to improving outcomes of IVF: consider the whole treatment E.M.E.W. Hum Reprod. 2004;19(9):1936–38. https://doi.org/10.1093/humrep/deh368
- Khan M, Zafar S, Syed S. Successful intravaginal culture of human embryos for the first time in Pakistan-an experience at the Sindh Institute of Reproductive Medicine, Karachi. JPMA. 2013;63(5):630–32. PMID: 23757995
- Coughlan CJ, Harrity C, Laird SM, Li TC. Local endometrial trauma (endometrial scratch): a treatment strategy to improve implantation rates. Sci Imp. 2016. Available from: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/local-endometrial-trauma-endometrial-scratch-a-treatment-strategy-to-improve-implantation-rates-scientific-impact-paper-no.-54/
- Tan J, Kan A, Hitkari J, Taylor B, Tallon N, Warraich G, et al. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J Assist Reprod Genet. 2018;35(4):683–92. https://doi.org/10.1007/s10815-017-1112-2
- Patel JA, Patel AJ, Banker JM, Shah SI, Banker MR. Personalized embryo transfer helps in improving in vitro fertilization/ICSI outcomes in patients with recurrent implantation failure. J Hum Reprod Sci. 2019;12(1):59–66. https://doi.org/10.4103/ jhrs.JHRS_74_18
- Baruffi RL, Mauri AL, Petersen CG, Nicoletti A, Pontes A, Oliveira JB, et al. Single-embryo transfer reduces clinical pregnancy rates and live births in fresh IVF and intracytoplasmic sperm injection (ICSI) cycles: a meta-analysis. Reprod Biol Endocrinol. 2009;23(7):36. https://doi.org/10.1186/1477-7827-7-36
- Igenomix. ERA procedure; 2021 Available from: https://www.igenomix.com/genetic-solutions/era-endometrial-receptivity-analysis
- Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28(1):14–38. https://doi.org/10.1016/j.rbmo.2013.08.011
- Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108(1):15–8. https://doi.org/10.1016/j.fertnstert.2017.05.033
- Simón C, Gomez C, Cabanillas S, Pellicer A, Mol BW, Valbuena D, et al. In vitro fertilization with personalized blastocyst transfer versus frozen or fresh blastocyst transfer: a multicenter randomized clinical trial. Fertil Steril. 2019;112(3):56–7. https://doi.org/10.1016/j.rbmo.2020.06.002
- Ruiz-Alonso M, Blesa D, Simón C. The genomics of the human endometrium. Biochem Biophys Acta. 2012;1822(12):1931– 42. https://doi.org/10.1016/j.bbadis.2012.05.004
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández- Sánchez M, Carranza F, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–24. https://doi.org/10.1016/j. fertnstert.2013.05.004
- Timeva T, Shterev A, Kyurkchiev S. Recurrent implantation failure: the role of the endometrium. J Reprod Infertil 2014;15(4):173–83. PMCID: PMC4227974
- Mahajan N. Endometrial receptivity array: clinical application. J Hum Reprod Sci. 2015;8(3):121–9. https://doi.org/10.4103/0974-1208.165153
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinol. 2006;147(3):1097–121. https://doi.org/10.1210/ en.2005-1076
- Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alama P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50–60. https://doi. org/10.1016/j.fertnstert.2010.04.063
- Ota T, Funabikia M, Tadaa Y, Karitaa M, Hayashia T, Maedaa K, et al. The reproductive outcomes for the infertile patients with recurrent implantation failures may be improved by endometrial receptivity array test. J Med Cases. 2019;10(5):138–40. https://doi.org/10.14740/jmc3282
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–95. https://doi.org/10.1056/NEJMp1500523
