Review Article
VOLUME: 38 | ISSUE: 3 | Sep 25, 2022 | PAGE: (129 - 133) | DOI: 10.51441/BioMedica/5-759
Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus
Authors:
Sidra Aslam
, Sarah Ghafoor
Article Info
Authors
Sidra Aslam
Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
Sarah Ghafoor
Assistant Professor, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
Publication History
Received: July 01, 2022
Revised: August 18, 2022
Accepted: September 03, 2022
Published: September 25, 2022
Abstract
Diabetes mellitus is a disorder of the metabolic system that is diagnosed by hyperglycemia and glucose intolerance. Diabetic patients are frequently prone to developing oral health complications, such as being at a higher risk for developing oral precancerous conditions like lichen planus, leukoplakia, and erythroplakia. Insulin-like growth factor-I (IGF-I) is a peptide hormone with a structure similar to insulin and belongs to the family of growth factors that play a vital role in the development of embryonic, postembryonic, and normal physiological functions of the human body. IGF-I, through autophosphorylation of its receptor, activates the mitogen-activated protein kinases pathway that leads to the expression of IGF-I hormone. In oral premalignant conditions, like lichen planus, submucous fibrosis, leukoplakia, and erythroplakia, the levels of the IGF-I hormone are increased. Subsequently, IGF-I can be used as a marker for early detection of malignancy in oral premalignant lesions.
Keywords: Insulin-like growth factor-I, diabetes mellitus, hyperglycemia, oral, premalignant lesions.
Pubmed Style
Sidra Aslam, Sarah Ghafoor. Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus. BioMedica. 2022; 25 (September 2022): 129-133. doi:10.51441/BioMedica/5-759
Web Style
Sidra Aslam, Sarah Ghafoor. Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus. https://biomedicapk.com/articles/online_first/759 [Access: July 03, 2024]. doi:10.51441/BioMedica/5-759
AMA (American Medical Association) Style
Sidra Aslam, Sarah Ghafoor. Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus. BioMedica. 2022; 25 (September 2022): 129-133. doi:10.51441/BioMedica/5-759
Vancouver/ICMJE Style
Sidra Aslam, Sarah Ghafoor. Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus. BioMedica. (2022), [cited July 03, 2024]; 25 (September 2022): 129-133. doi:10.51441/BioMedica/5-759
Harvard Style
Sidra Aslam, Sarah Ghafoor (2022) Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus. BioMedica, 25 (September 2022): 129-133. doi:10.51441/BioMedica/5-759
Chicago Style
Sidra Aslam, Sarah Ghafoor. "Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus." 25 (2022), 129-133. doi:10.51441/BioMedica/5-759
MLA (The Modern Language Association) Style
Sidra Aslam, Sarah Ghafoor. "Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus." 25.September 2022 (2022), 129-133. Print. doi:10.51441/BioMedica/5-759
APA (American Psychological Association) Style
Sidra Aslam, Sarah Ghafoor (2022) Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus. , 25 (September 2022), 129-133. doi:10.51441/BioMedica/5-759
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 38(3):129-133
REVIEW ARTICLE
Insulin-like growth factor-I signaling plays a pivotal role in the development of oral premalignant lesions in patients with diabetes mellitus
Sidra Aslam1, Sarah Ghafoor2* 
Received: 01 July 2022 Revised date: 18 August 2022 Accepted: 03 September 2022
Correspondence to: Sarah Ghafoor
*Assistant Professor, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan.
Email: sarahghafoor@uhs.edu.pk
Full list of author information is available at the end of the article.
ABSTRACT
Diabetes mellitus is a disorder of the metabolic system that is diagnosed by hyperglycemia and glucose intolerance. Diabetic patients are frequently prone to developing oral health complications, such as being at a higher risk for developing oral precancerous conditions, like lichen planus, leukoplakia, and erythroplakia. Insulin-like growth factor-I (IGF-I) is a peptide hormone with a structure similar to insulin and belongs to the family of growth factors that play a vital role in the development of embryonic, postembryonic, and normal physiological functions of the human body. IGF-I, through autophosphorylation of its receptor, activates the mitogen-activated protein kinases pathway that leads to the expression of IGF-I hormone. In oral premalignant conditions, like lichen planus, submucous fibrosis, leukoplakia, and erythroplakia, the levels of the IGF-I hormone are increased. Subsequently, IGF-I can be used as a marker for early detection of malignancy in oral premalignant lesions.
Keywords:
Insulin-like growth factor-I, diabetes mellitus, hyperglycemia, oral, premalignant lesions.
Introduction
Diabetes mellitus is a disorder of the metabolic system which is diagnosed by chronic hyperglycemia and intolerance to glucose which is either the result of deficiency of insulin or impaired action of insulin or both. Diabetic patients are frequently prone to developing oral health infections. These infections include periodontitis, gingivitis, and angular chelitis. In addition to this, diabetes may act as a potent etiological factor for developing oral leukoplakia, erythroplakia, and oral lichen planus (OLP).1,2
The insulin-like growth factor (IGF) is a family that consists of three ligands, three receptors and IGF-binding proteins. The ligands include peptide hormones such as insulin, IGF-I, and IGF-II. The three receptors are insulin receptor, IGF-I receptor (IGF-IR), and IGF-II receptor or mannose-6-phosphate. This signaling has a significant role to play in the embryonic as well as postembryonic developmental period and is a vital molecule in the maintenance of normal physiology of an adult.3 IGF-I and IGF-II regulate proliferation of cell, cell differentiation, and apoptosis. IGF-I is a single chain 70 amino acid, nonglycosylated peptide, weighs about 7.6 kD and has four domains, known as A, B, C, and D. IGF-I has a structure similar to insulin. The IGF-I that circulates in the body is secreted mainly from the liver, but some other tissues, namely fat tissues, also contribute to the secretion. Growth hormone and insulin regulate its biosynthesis from the liver (Figure 1).4
When IGF-I binds to its receptor, it results in the autophosphorylation of its receptor. Autophosphorylation occurs on three chains of tyrosine and activates the intrinsic tyrosine kinase activity of the IGF-IR. This activated IGF-IR then phosphorylates other substrates that contain tyrosine. Insulin receptor substrate-1 (IRS-1) is the predominant isoform of this hormone. Multiple tyrosine domains are located in YMXM motif of IRS-1 that are linked with proteins that contain Src homology 2 (SH2) domains like phosphoinositol 3’ (P13) kinase, growth factor receptor-bound protein-2 (Grb-2), noncatalytic region of tyrosine kinase adaptor protein, and synaptophysin. IRS-1 adapter protein can therefore bring these proteins together, which contain SH2 domains and regulate their activity. When IGF-IR is activated, it also causes the phosphorylation of the Shc adapter protein. The Shc protein then couples with Grb-2 protein. Grb-2 contains SH2 and SH3 domains and is linked to mSos, which is guanine nucleotide exchange protein that converts the Ras-Guanosine diphosphate (GDP) into its active form. In turn, the activated Ras-GDP activates a cascade of reactions, which leads to mitogen-activated protein (MAP) kinase. The MAP kinases activate the nuclear transcription which ultimately leads to the expression of the IGF-I gene. Mutations within the expressions of this IGF-I gene leads to a spectrum of disorders of growth hormone in humans such as growth hormone insensitivity syndrome and Laron syndrome.5 Some of the features of Laron syndrome include short limbs, frontal bossing, hypoplastic maxilla and mandible, malocclusion of teeth, and increased enamel thickness.6
IGF-I has a vital part in the maintenance of immune functions and controls the proliferative activity of immune cells, cellular differentiation, apoptosis, and autophagy by binding to IGF-IR. Conversely, IGF-I prevents the apoptosis pathway by stimulating the release of cytokines that are anti-apoptotic in nature. After the activation of IGF-IR, IGF initiates the phosphorylation of two pathways: extracellular signal-regulated kinase (ERK) pathway and phosphoinositide 3-kinase/mammalian target of rapamycin (PI3K/mTOR) pathway. These two pathways play an important role in autophagy. ERK pathway stimulates autophagy, but the stimulation of P13K/mTOR pathway inhibits autophagy (Figure 2).7 Due to this, IGF-I has been linked to the pathological transformations observed in oral premalignant conditions, such as lichen planus, leukoplakia, erythroplakia, and submucous fibrosis.7 This narrative review discusses the increased incidence of oral leukoplakia and erythroplakia, along with the OLP among patients of diabetes mellitus and the relationship with IGF-1 signaling in oral premalignant lesions. Electronic literature databases, such as PubMed and Google Scholar, were searched using the Medical Subject Headings terms such as IGF-I, diabetes mellitus, oral leukoplakia, erythroplakia, OLP, and oral submucous fibrosis (OSMF) in different combinations. All research papers in English language having full-texts available were accessed and included in this review. Research papers published in languages other than English and whose full texts were not available were excluded from the study.
Diabetes Mellitus and Oral Premalignant Lesions
Correlations between diabetic patients and oral inflammatory and precancerous lesions, like oral leukoplakia, erythroplakia, and OLP, has been a well-established fact.8,9 In diabetes mellitus, the breakdown of oxidative equilibrium occurs resulting in elevation of glucose, abundant formation of oxygen free radicals, and the glycation of proteins that depress the activity of antioxidant enzymes. This process leads to significant damage in the biological structure of pancreas at the molecular level. The oxygen free radicals and oxidative stress have an important function in the progression of oral premalignant conditions, such as leukoplakia and erythroplakia, toward malignancy.10 Individuals with diabetes are at increased risk for periodontal disease, salivary gland dysfunction, dental caries, mucosal abnormalities, and oral burning, all of which can negatively impact patient quality of life. The bidirectional relationship between diabetes and periodontal disease is of particular importance due to the negative effect of periodontitis on glycemic control and the potential benefit of periodontal therapy on glycemic control. A study conducted in Hungary also suggested a link between diabetic patients and leukoplakia and erythroplakia.11 Another study conducted in India reported a correlation between diabetes mellitus and developing leukoplakia. This study showed that hyperglycemia increases the chances of malignancy in the oral variant of leukoplakia.12 A high prevalence of oral leukoplakia has been reported in diabetic patients as compared to nondiabetics according to a study in China.13 An association has been suggested between OLP and diabetes mellitus that could be due to the dysfunction in endocrine system in diabetes mellitus that may be due to an immune-deficiency and thus contributes in the progression of OLP.13 Another study conducted among the Pakistani population indicated that diabetics were 2.5 times more likely to develop OLP than nondiabetics. This study concluded that a significant association exists between diabetes mellitus and developing OLP, with a female predilection and buccal mucosa as the most commonly affected sites among diabetic subjects. 14
IGF-I and Oral Submucous Fibrosis
OSMF is a precancerous lesion of the mouth which is identified by the inflammatory condition and fibrosis of the oral mucosal tissues resulting in trismus, which is limited mouth opening.15,16 A study reported that IGF-I directly stimulates the proliferation of fibroblasts and synthesis of collagen which is important in the fibroproliferative process of OSMF. When normal buccal mucosa and OSMF buccal mucosa specimens obtained from cell culture were compared, it was found that very minute levels of IGF-I were present in normal buccal mucosa samples, while in OSMF samples, an increased expression of IGF-I was seen in the cytoplasm of fibroblasts, inflammatory cells, and endothelial cells.17,18 A study conducted among the Indian population reported that prevalence of erythroplakia among diabetes mellitus patients was 3.2%, while in case of OSMF, a nonsignificant and inverse relationship between OSMF and diabetes mellitus was recorded.9
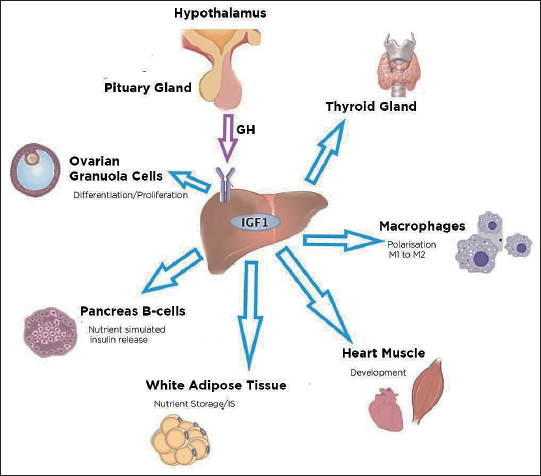
Figure 1. Actions of IGF-I on different tissues of the human body. IGF-1 is synthesized in the liver under the action of growth hormone from the hypothalamus. In the thyroid gland, it regulates hematopoiesis and immune cell function. IGF-1 promotes the differentiation of macrophages. In the cardiovascular system, IGF-1 acts as a vasodilator and helps in the development and protection of heart muscles. IGF-1 has an essential role in stimulating cell proliferation, differentiation, and lipid accumulation. IGF-1 stimulates the release of insulin from B-cells of the pancreas. IGF-1 also promotes ovarian folliculogenesis and testicular function and integrity. IGF-1, M1, and M2: phenotypes of macrophages. Blue arrows represent the role of IGF-1 on the different tissues of the human body.
IGF-I and oral lichen planus
OLP is a potentially malignant disorder and a disease of the immune system which is identified by infiltrating T lymphocytes in the lamina propria associated with degenerating keratinocytes and disrupting basement membrane of the oral mucosa. Studies have documented that OLP patients have disturbances in glucose metabolism and show increased expressions of IGF-1R in lesional tissue as compared with controls. It is, therefore, speculated that IGF-I signaling may participate in the immune-regulatory mechanisms concerned with OLP.19-21
IGF-I and oral leukoplakia
Oral leukoplakia is a white patch or plaque on the inside of mouth that cannot be characterized clinically or pathologically as any other disease.22 Increased IGF-IR has shown to be associated with tumor cell proliferation, migration, invasion, and metastasis. In a study conducted in Turkey, biopsy samples of 38 patients of oral squamous cell carcinoma (OSCC), 32 patients having leukoplakia of oral cavity and 15 patients with oral fibrous hyperplasia were selected. The results of this study demonstrated an elevated expression of IGF-IR in OSCC and oral leukoplakia samples, thus suggesting an increased expression of IGF-IR in neoplastic cell proliferation and malignancy.23 Subsequently, IGF-IR can be used as a marker for early detection of malignancy.24
IGF-I and oral erythroplakia
Erythroplakia is characterized by any lesion of the oral mucosa that presents a bright red velvety patch that cannot be characterized clinically or pathologically as any other recognizable condition.25 A study conducted among the Indian population determined the levels of IGF-I in patients diagnosed with erythroplakia and OSCC. The study concluded that positive correlation existed between increased IGF-I levels and premalignant lesions like erythroplakia. As the expression of IGF-I protein was found to be elevated in erythroplakia as compared to OSCC, this study indicated that serum IGF-I levels were increased in conditions which have a better prognosis such as erythroplakia and OSCC stage I, whereas the levels of IGF-I protein were downregulated in conditions with poor prognosis, such as OSCC stage IV.26
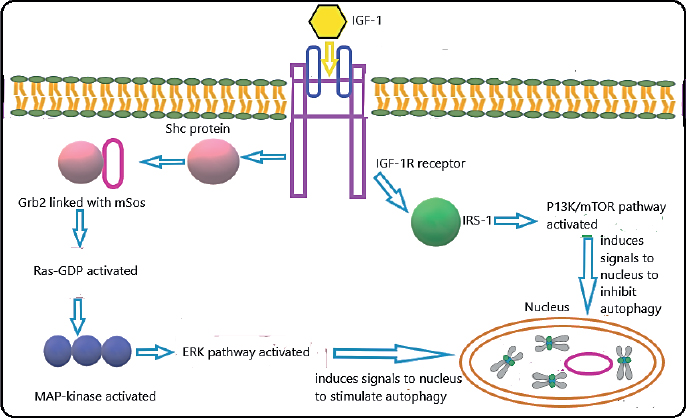
Figure 2. Dual role of IGF-I in stimulation and inhibition of autophagy. The key signaling pathways have been discussed in this review. IGF-I can activate ERK and P13K/mTOR signaling pathways that are involved in the stimulation and inhibition of autophagy, respectively, IGF-I and IRS.
Conclusion
A higher incidence of oral leukoplakia, erythroplakia, and OLP has been reported in diabetic patients as compared to nondiabetic individuals, and thus early diagnosis and treatment planning of diabetes mellitus can reduce the risk of developing oral premalignant lesions. IGF-I is also associated with oral premalignant lesions. Regular screening of diabetes mellitus and regular examination of oral cavity can help clinicians as well as patients in early identification of any underlying oral pathology.
Acknowledgement
The authors are grateful to the Higher Education Commission for providing e-library access to the University of Health Sciences (UHS), Lahore, for acquisition of data for this review paper.
List of Abbreviations
| ERK | Extracellular signal-regulated kinase |
| Grb-2 | Growth factor receptor bound protein 2 |
| IGF-I | Insulin-like growth factor-I |
| IGF-II | Insulin-like growth factor-II |
| IGF-IR | Insulin-like growth factor-I receptor |
| IRS-1 | Insulin receptor substrate 1 |
| MAP kinase | Mitogen-activated protein kinase |
| OLP | Oral lichen planus |
| OSMF | Oral submucous fibrosis |
| OSCC | Oral squamous cell carcinoma |
| SH2 | Src homology 2 |
Conflict of interest
None to declare.
Grant support and financial disclosure
The M. Phil research of the first author has been fully funded by the UHS, Lahore, Pakistan.
Ethical approval
Not required.
Authors’ contributions
SA: Acquisition of published data and manuscript writing.
SG: Conception of the study, critical revisions through intellectual content, and approval of the final version of the manuscript to be published.
Authors’ details
Sidra Aslam1, Sarah Ghafoor2
- Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
- Assistant Professor, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
References
- Thayumanavan B, Jeyanthikumari T, Abu Dakir, Vani NV. Diabetes and oral health-an overview of clinical cases. Int J Med Dent Sci. 2015;4(2):901–5. https://doi.10.19056/ijmdsjssmes/2015/v4i2/79864
- Mohsin SF, Ahmed SA, Fawwad A, Basit A. Prevalence of oral mucosal alterations in type 2 diabetes mellitus patients attending a diabetic center. Pak J Med Sci. 2014;30(4):716–9. https://doi.10.12669/pjms.304.5220
- Roith DL. The insulin-like growth factor system. J Diabetes Res. 2003;4(4):205–12. https://doi.10.1155/EDR.2003.205
- Le Roith D, Scavo L, Butler A. What is the role of circulating IGF-I? Trends Endocrinol Metab. 2001;12(2):48–52. https://doi.10.1016/S1043-2760(00)00349-0
- LeRoith D, Werner H, Beitner-Johnson D, Roberts Jr CT. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16(2):143–63. https://doi.10.1210/edrv-16-2-143
- Mungan NC. Laron syndrome: a case report. Aydın Dent J. 2017;3(2):45–8.
- Wang F, Tan YQ, Zhang J, Zhou G. Insulin-like growth factor 1 exhibits the pro-autophagic and anti-apoptotic activity on T cells of oral lichen planus. Int J Biol Macromol. 2019;133:640–6. https://doi.10.1016/j.ijbiomac.2019.04.158
- Vairaktaris E, Spyridonidou S, Goutzanis L, Vylliotis A, Lazaris A, Donta I, et al. Diabetes and oral oncogenesis. Anticancer Res. 2007;27(6):4185–93.
- Dikshit RP, Ramadas K, Hashibe M, Thomas G, Somanathan T, Sankaranarayanan R. Association between diabetes mellitus and pre-malignant oral diseases: a cross sectional study in Kerala, India. Int J Cancer Res. 2006;118(2):453–7. https://doi.10.1002/ijc.21345
- Kumar S, Debnath N, Ismail MB, Kumar A, Kumar A, Badiyani BK, et al. Prevalence and risk factors for oral potentially malignant disorders in Indian population. Int J Prev Med. 2015;2015:208519. https://doi.10.1155/2015/208519
- UJPal M, Matos O, Bibok G, Somogyi A, Szabó G, Suba Z. Diabetes and oral tumors in Hungary: epidemiological correlations. Diabetes Care. 2004;27(3):770–4. https://doi.10.2337/diacare.27.3.770
- Li J, Liu Y, Zhang H, Hua H. Association between hyperglycemia and the malignant transformation of oral leukoplakia in China. Oral Dis. 2020;26(7):1402–13. https://doi.10.1111/odi.13372
- Dean D, Gandara B. Oral manifestations of diabetes. In: Poretsky L, editor. Principles of diabetes mellitus. Cham, Switzerland: Springer. https://doi.10.1007/978-3-319-18741-9_54.
- Ahmed I, Nasreen S, Jehangir U, Wahid Z. Frequency of oral lichen planus in patients with noninsulin dependent diabetes mellitus. J Pak Assoc Dermatol. 2012;22(1):30–4.
- Passi D, Bhanot P, Kacker D, Chahal D, Atri M, Panwar Y. Oral submucous fibrosis: newer proposed classification with critical updates in pathogenesis and management strategies. Natl J Maxillofac Surg. 2017;8(2):89–94. https://doi. 10.4103/njms.NJMS_32_17
- Arakeri G, Rai KK, Hunasgi S, Merkx MAW, Gao S, Brennan PA. Oral submucous fibrosis: an update on current theories of pathogenesis. J Oral Pathol Med. 2017;46(6):406–12. https://doi.10.1111/jop.12581
- Tsai CH, Yang SF, Chen YJ, Chou MY, Chang YC. The upregulation of insulin-like growth factor-1 in oral submucous fibrosis. Oral Oncol. 2005;41(9):940–6. https://doi. 10.1016/j.oraloncology.2005.05.006
- Olson MA, Rogers III RS, Bruce AJ. Oral lichen planus. Clin Dermatol. 2016;34(4):495–504. https://doi. 10.1016/j.clindermatol.2016.02.023
- Tan YQ, Zhang J, Du GF, Lu R, Chen GY, Zhou G. Altered autophagy-associated genes expression in T cells of oral lichen planus correlated with clinical features. Mediators Inflamm. 2016;2016:4867368. https://doi.10.1155/2016/4867368
- Ma RJ, Tan YQ, Zhou G. Aberrant IGF1-PI3K/AKT/MTOR signaling pathway regulates the local immunity of oral lichen planus. Immunobiology. 2019;224(3):455–61. https://doi.10.1016/j.imbio.2019.01.004
- Seyhan M, Özcan H, Sahin I, Bayram N, Karincaoğlu Y. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77(2):198–202. https://doi.10.1016/j.diabres.2006.12.016
- Shanbhag VKL. New definition proposed for oral leukoplakia. Dent Res J (Isfahan). 2017;14(4):297–8. https://doi.10.4103/1735-3327.211627
- Mutlu GS, Dölek GS, Kayhan K, Namdar PF, Başaran B, Alatlı F. Expressions of IGF-1R, EZH2, laminin-5 in leukoplakia and oral squamous cell carcinoma. Ata DişHekFakDerg. 2021;31(2):241–6. https://doi.10.17567/ataunidfd.890469
- Biamonte F, Buffone C, Santamaria G, Battaglia AM, Mignogna C, Fortunato L, et al. Gene expression analysis of autofluorescence margins in leukoplakia and oral carcinoma: a pilot study. Oral Dis. 2021;27(2):193–203. https://doi.10.1111/odi.13525
- Langdon JD. Malignant disease of the oral cavity. Oral Maxillofac Surg. 2007;127–44. https://doi.10.1016/B978-0-443-10073-4.50014-8
- Tiwari SK, Saini S, Singhal P, Mathur A, Sinha M. The diagnostic and prognostic utility of insulin growth factor of squamous cell carcinoma in oral cavity. Tzu Chi Med J. 2021;33(2):160–4. https://doi.10.4103/tcmj.tcmj_50_20
