Original Article
VOLUME: 37 | ISSUE: 2 | Jun 30, 2021 | PAGE: (100 - 104) | DOI: 10.51441/BioMedica/5-281
Lack of Association between CYP1A1 3801T > C Polymorphism and Idiopathic Male Infertility in Algerian Population
Authors: Chellat Djalila , Rezgoun Mohamed Larbi , Mcelreavey Kenneth , Abadi Norreddine , Satta Dalila
Article Info
Authors
Chellat Djalila
Laboratory of Molecular and Cellular Biology, University Frères Mentouri Constantine 1, Constantine - Algeria.
Rezgoun Mohamed Larbi
Laboratory of Molecular and Cellular Biology, University Frères Mentouri Constantine 1, Constantine - Algeria.
Mcelreavey Kenneth
Department of Human Developmental Genetics, Pasteur Institute, Paris - France.
Abadi Norreddine
Laboratory of Biology and Molecular Genetics, Hospital Center University Ben-Badis, Constantine - Algeria.
Satta Dalila
Laboratory of Molecular and Cellular Biology, University Frères Mentouri Constantine 1, Constantine - Algeria.
Publication History
Received: February 10, 2021
Revised: April 22, 2021
Accepted: May 26, 2021
Published: June 30, 2021
Abstract
Background and Objective: Ongoing research suggests that cytochrome P4501A1 (CYP1A1) 3801T > C polymorphism may be correlated with human male infertility but the reported results are conflicting. Hence, this case-control study was conducted in Algerian population to determine the frequency of this polymorphism and its relationship to male infertility.
Methods: This study included 173 subjects grouped into two categories: controls (84) and patients (89) with abnormal semen analysis parameters. Genomic DNA from the patients and controls was extracted and PCR-restriction fragment length polymorphism was used to genotype the 3801T > C CYP1A1 polymorphism.
Results: In the control group, the frequency of homozygous wild-type TT, heterozygous TC, and mutant homozygous CC genotypes of the CYP1A1 T > C polymorphism was 84.52%, 13.10%, and 2.38%, respectively, while infertile men had 77.53%, 20.22%, and 2.25%, respectively. There was no correlation between the 3801T > C CYP1A1 variant and male infertility. Furthermore, the rs4646903 C allele was not a risk factor in the dominant genetic model.
Conclusion: The 3801T > C polymorphism cannot be considered as a risk factor for male infertility in Algerian population. Our results need to be validated and confirmed through prospective studies with a larger number of patients.
Keywords: Male infertility; CYP1A1, 3801T>C polymorphism; PCR/Digestion.
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(2):100-104
ORIGINAL ARTICLE
Lack of association between CYP1A1 3801T > C polymorphism and idiopathic male infertility in Algerian population
Chellat Djalila1,2*, Rezgoun Mohamed Larbi1,2, Mcelreavey Kenneth3, Abadi Norreddine2, Satta Dalila1,2
Received: 10 February 2021 Revised date: 22 April 2021 Accepted: 26 May 2021
Correspondence to: Chellat Djalila
Laboratory of Molecular and Cellular Biology, University Frères Mentouri Constantine 1, Constantine, Algeria.
Email: rezgoune.chellat.djalila@umc.edu.dz
Full list of author information is available at the end of the article.
ABSTRACT
Backgrounds and Objective:
Many studies suggested that cytochrome P4501A1 (CYP1A1) 3801T > C polymorphism may be correlated with human male infertility but the reported results are conflicting. Hence, we conducted this case-control study in Algerian population to determine the frequency of this polymorphism and its relationship to male infertility.
Methods:
This study included 173 subjects grouped into two categories: controls (84) and patients (89) with abnormal semen analysis parameters. Genomic DNA from the patients and controls was extracted and PCR-restriction fragment length polymorphism was used to genotype the 3801T > C CYP1A1 polymorphism.
Results:
In the control group, the prevalence of homozygous wild-type TT, heterozygous TC, and mutant homozygous CC genotypes of the CYP1A1 T > C polymorphism was 84.52%, 13.10%, and 2.38%, respectively, while infertile men had 77.53%, 20.22%, and 2.25%, respectively. There was no correlation between the 3801T > C CYP1A1 variant and male infertility. Furthermore, the rs4646903 C allele was not a risk in the dominant genetic model.
Conclusion:
The 3801T > C polymorphism cannot be considered as a risk factor for male infertility in Algerian population. Our results need to be validated and confirmed through prospective studies with a larger number of patients.
Keywords:
Male infertility, CYP1A1, 3801T > C polymorphism, PCR/digestion, Algeria.
Introduction
The World Health Organization (WHO) estimates that male infertility accounts for about half of all infertility cases and affects 9% of couples worldwide [1]. The etiologies of this disorder are the product of a complex gene-gene and/or gene-environment interaction [2].
Genetic abnormalities account for 15%-30% of male infertility cases [3]. Furthermore, environmental factors such as polycyclic aromatic hydrocarbons (PAHs) are endocrine disruptors, and their activated metabolite may form DNA adducts which may be linked to a drop in human sperm quality [4].
Cytochrome P4501A1 (CYP1A1) is a crucial enzyme in phase I oxidation reactions that catalyze a wide variety of environmental toxins and carcinogens [5]. CYP1A1 gene is positioned on chromosome 15q22-q24, encodes a 512 amino acid protein and it is expressed in the liver, gastrointestinal tract, lymphocytes, and testicular tissue [6]. Several CYP1A1 gene polymorphisms have been identified [7], but the 3801T > C variant has received the most attention due to its higher genotype frequency and potential role in spermatogenesis. CYP1A1 3801T > C polymorphism is a T to C base substitution in the 3’-untranslated region (3’UTR) [7,8]. CYP1A1 is implicated in the synthesis of endogenous substrates including steroid hormones, and the variant may be linked to the production of highly reactive electrophilic intermediates that could damage DNA in the meiotic division during spermatogenesis, diminish semen quality and lead to male infertility [9].
In recent years, several studies proposed that CYP1A1 3801T > C polymorphism may be associated with spermatogenic failure causing infertility, but the findings from previous studies reported conflicting results [10-12].
The aim of this study was to determine the relationship between the CYP1A1 3801T > C polymorphism and the possibility of human male infertility in the Algerian population.
Methods
Study population
This case-control study included 173 subjects comprising 89 infertile patients and 84 controls. Patients were diagnosed at Ibn Sina Laboratory (8 Rue Belhoula El Mekki Constantine, Algeria) and Ibn Rochd Clinic (BOUSSOUF Abdelhafid city, Constantine, Algeria) between June 2012 and May 2018. After receiving written informed consent, all of the participants were personally interviewed. Controls and patients were matched for age and geographic origin. The infertile men with unexplained etiology (idiopathic) were included while those with known causes (cytogenetic, biochemical, and Y-chromosomal deletions) were excluded.
Sperm count test was performed as per WHO guidelines, 2010 [13] by a trained technician. The reference values for sperm parameters were: ≥1.5 for semen volume, ≥15 × 106 ml-1 for sperm concentration, ≥39 × 106 per ejaculate for total sperm counts, ≥58% for vitality, ≥40% for total motility, ≥32% for sperm progressive motility, ≥4% for normal morphology.
The control group consisted of 80 men who had already fathered at least one child without the use of assisted reproductive technology.
Genotyping
Genomic DNA was extracted using the salting-out procedure from peripheral blood leukocytes. PCR-restriction fragment length polymorphism (PCR-RFLP) was used to genotype the polymorphism.
The PCR was carried out in 20 μl. Polymerase reaction consisted of a buffer (10×), Deoxyribonucleotide triphosphate (dNTP) (10 mM), Taq polymerase (5 unit/μl), and primers. The product size of the 3’UTR of CYP1A1 gene was 340 bp. The following primer sequences were used: forward 5’-GGCTGAGCAATCTGACCCTA-3’ and reverse 5’-TAGGAGTCTTGTCTCATGCCT-3’. Polymerase Chain Reaction (PCR) conditions used for amplification of CYP1A1 gene consisted of initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 61°C for 30 seconds and extension at 72°C for 30 seconds with a final extension at 72°C for 4 minutes. 10 μl of the products were electrophoresed at 100 V on a 2% agarose gel.
A 340-bp fragment was obtained after amplification; Each PCR product (10 μl) was subjected to Moraxella species (Msp1) digestion at 37°C for 12 hours and analyzed by gel electrophoresis (3%). The occurrence of a polymorphic Msp1 restriction site results in 200-bp and 140-bp fragments, suggesting that the C allele is present (CYP1A1 3801T > C) (Figure 1).
Statistical analysis
To see whether the genotype frequencies in the patient and control samples were statistically different, a probability of p < 0.05 was considered to be significant. The Hardy-Weinberg Equilibrium (HWE) departure for each group was tested using the Pearson χ2 test in the online HWE software (http://ihg2.helmholtzmuenchen.de/cgi-bin/hw/hwa1.pl).
Results
This research was designed to determine the effect of 3801T > C CYP1A1 polymorphism in the development of abnormal sperm parameters. This variant was selected because of its role in metabolism of fertility hormones and xenobiotic substances/endocrine disrupters, known to influence the male fertility. According to semen analysis, infertile patients were divided into 3 groups: azoospermic (AZOs) group (absence of spermatozoa in the ejaculate), asthenospermic (ASTs) (low sperm motility), teratozoospermic (abnormal sperm form), and Oligoasthenoteratospermic (OATs) group (condition that comprises of low number, poor motility and abnormal forms of spermatozoa). The distribution of genotype and allele frequency of 3801T > C polymorphism is detailed in Table 1. No significant association for male infertility was found for CYP1A1 3801T > C polymorphism (TT, TC, and CC) in infertile patients (77.53%, 20.22%, and 2.25%) versus fertile controls (84.52%, 13.10%, and 2.38%). No significant associations between the TC and CC genotypes and AZOs (OR = 1.37, 95% CI = 0.49-3.83, p = 0.66; OR = 1.51, 95% CI = 0.15-15.68, p = 0.91, respectively), OATs (OR = 2.58, 95% CI = 0.71-9.22, p = 0.18; OR = 0, 95% CI = 0-21.43, p = 0.76, respectively) and ASTs (OR = 1.84 95% CI = 0.23-11.91, p = 0.83; OR = 0, 95% CI = 0-51.53, p = 0.41) were noted.
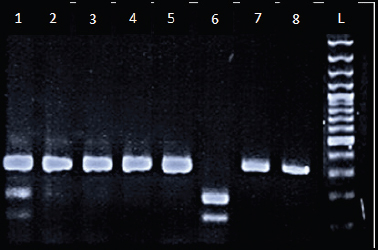
Figure 1. CYP1A1 gene polymorphism was analyzed by PCR-RFLP. Lanes L; Ladder (100 bp); Lanes 1; Heterozygous genotype (CT), Lanes 2-5 and 7-8; Wild homozygous (TT), and Lane 6; Mutant homozygous (CC).
Statistical analysis revealed that the genotypes of the polymorphism studied were in Hardy-Weinberg equilibrium in both infertile and fertile groups (p > 0.05).
Discussion
CYP1A1 belongs to the CYP1 family of enzymes and is involved in the synthesis of a wide range of xenobiotics, endogenous substrates, and steroidal hormones, including estrogens [9]. The bioactivation of PAHs is catalyzed by cytochrome P450s, which are the phase I enzymes. PAHs are common contaminants in the natural environment that can form DNA adducts when triggered, resulting in DNA reactive metabolite [14]. Changing the level of gene expression or the stability of messenger RNA, polymorphisms of CYP1A1 may cause damage to DNA, resulting in highly inducible enzyme activity [8].
CYP1A1 T to C base transition gives rise to a MspI restriction site. After the discovery of the CAYP1A1 polymorphisms, a number of studies in different ethnic communities have been performed to investigate the role of CYP1A1 polymorphisms in male infertility [2]. However, no such research has been conducted in the Algerian population to-date.
This case-control analysis was conducted to investigate the possible association between the CYP1A1 3801T > C polymorphism and male infertility risk. In allele comparison, homozygous, and recessive genetic models, the 3801T > C variant was found to be unrelated to the male infertility. The same findings were obtained in analyses of infertile subjects with AZOs, OATs, and ASTs (Table 2).
These results are coherent with previous research in a variety of ethnic groups. In studies of North Iranian populations, the 3801T > C of CYP1A1 was found to have no association with male infertility [15]. In addition, CYP1A1 variants had no effect on disease vulnerability in the Russian population, according to Yarosh et al. [16] Lu et al. [17] have found no significant correlation between the 3801T > C polymorphism and male infertility, but CYP1A1*2C polymorphism may conduce to male infertility in the Han-Chinese population. Similarly, the allele frequency of the 3801T > C polymorphism does not correlate with semen production in the American population (New Jersey), according to Franasiak et al. [10].
Table 1. Distribution of genotype/allele frequency of CYP1A1 3801 T > C in association with male infertility.
| Genotypes | Patients (infertile) % (N) | Controls % (N) | OR | p value |
|---|---|---|---|---|
| 3801TT | 77.53% (69) | 84.52% (71) | Ref | - |
| 3801TC | 20.22% (18) | 13.10% (11) | 1.68 [0.69-4.14] | 0.29 |
| 3801CC | 2.25% (02) | 2.38% (02) | 1.03 [0.10-10.58] | 0.63 |
| TC + TT vs. TT | 22.47% (20) | 15.48% (13) | 1.58 [0.69-3.69] | 0.32 |
| TT + TC vs. CC | 97.75% (87) | 97.62% (82) | 1.06 [0.10-10.83] | 0.65 |
| T allele | 87.64% (156) | 91.07% (153) | Ref | - |
| C allele | 12.36% (22) | 8.93% (15) | 1.44 [0.68-3.04] | 0.39 |
Ref: reference.
Table 2. The frequency of the CYP1A1 3801TC genotype according to the three subtypes of the infertile male group.
| TT | TC | CC | TC + CC | C | |
|---|---|---|---|---|---|
| AZOs % | 79.66% | 16.95% | 03.39% | 20.34% | 11.86% |
| n = 59 | n = 47 | n = 10 | n = 02 | n = 12 | n = 14 |
| OR (95 % CI) | / | 1.37 [0.49-3.83] | 1.51 [0.15-15.68] | 1.39 [0.54-3.60] | 1.37 [0.60-3.16] |
| p | / | 0.66 | 0.91 | 0.59 | 0.54 |
| OATs % | 71.43% | 28.57% | 0% | 28.57% | 14.29% |
| n = 21 | n = 15 | n = 6 | n = 0 | n = 6 | n = 6 |
| OR (95 % CI) | / | 2.58 [0.71-9.22] | 0 [0-21.43] | 0.62 [0.62-7.55] | 1.70 [0.54-5.11] |
| p | / | 0.18 | 0.76 | 0.28 | 0.45 |
| ASTs % | 77.78% | 22.22% | 0% | 22.22% | 11.11% |
| n = 09 | n = 7 | n = 2 | n = 0 | n = 2 | n = 2 |
| OR (95 % CI) | / | 1.84 [0.23-11.91] | 0 [0-51.53] | 1.56 [0.20-9.80] | 1.27 [0-6.69] |
| p | / | 0.83 | 0.41 | 0.96 | 0.90 |
| Controls % n = 84 |
84.52 n = 71 |
13.10 n = 11 |
2.38 n = 02 |
15.48 n = 13 |
8.93 n = 15 |
AZOs: Azoospermics; OR: Odds Ratio; CI: Confidence Interval; OATs: Oligoasthenoteratospermics; ASTs: Asthenospermics.
Our findings contradict those of Vani et al. [9], who observed variations in sperm characteristics in both infertile and fertile men (controls) with the TT, TC, and CC genotypes, and that these differences were major except for semen volume and total sperm count. According to Wang et al. [18] and Ramgir et al. [19], this polymorphism (p = 0.040) may lead to the pathogenesis of male infertility in Chinese and South Indian population.
A meta-analysis carried out by Fang et al. [20] conclude that the CYP1A1 3801T > C variant was found to be associated with increased risk of male infertility in Asians but not in Caucasians. Moreover, Luo et al. [2] performed a meta-analysis and found an important association between the CYP1A1 3801T > C polymorphism and the possibility of idiopathic male infertility. Nejati et al. [21] have conducted a meta-analysis and have indicated that, CYP1A1 3801T > C transition can be considered as a biomarker for risk of male infertility within Asian populations. Cao et al. [22] have similarly investigated the relationship between the studied polymorphism and male infertility risk and came to the conclusion that this variant can play a role in individual susceptibility to male infertility in Asians.
The inconsistency of the findings from different studies may be attributed to a variety of reasons, including variations in the sample populations' recruiting processes and genetic and environmental backgrounds.
Our negative results may be due to the limited sample size, the fact that this region is highly conserved, or the fact that the CYP1A1 3801T > C polymorphism has no effect on male infertility risk. Furthermore, the low penetrance genetic consequences of a single polymorphism can be heavily influenced by interactions with other polymorphisms and/or a specific environmental exposure.
The biological mechanism by which CYP1A1 influences the pathogenesis of male infertility is still unknown. According to Vani et al. [9], DNA adducts in sperm cells can be a sign of significant DNA disruption, which can reduce the quality of sperm and lead to male infertility. The toxicity of PAHs on spermatogenic cells that undergo meiotic division has been demonstrated in vitro using a rat model, according to Georgellis et al. [23].
Conclusion
CYP1A1 3801TC polymorphism is not a risk factor for idiopathic male infertility in Algerian men. Further studies are however strongly suggested involving larger number of patients and other phase II gene polymorphisms.
Limitations of study
The first limitation of this study is the small sample size. Second, no information about the patients' behaviors, such as smoking or other potentially harmful activities was investigated. A selection bias may have occurred in this study influencing the identification of real associations. Additionally, only one gene involved in the detoxification was studied and no study of gene-environment interactions was done. Further studies with a larger sample size and different genes of phase I and phase II detoxification are needed to confirm these findings.
Acknowledgement
The authors would like to thank all members of Biology and Molecular Genetics Laboratory Hospital Center University Ben-Badis, Constantine, Algeria for DNA extraction. The authors also thank all patients and controls for sample contribution.
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose.
Ethical approval
The work received approval from the Ethics Committee of the Benbadis-Constantine University Hospital Centre, Algeria, institutes' local ethics committee vide Letter No. CHU-CONST-05/18-2-2016. For the study, all participants signed a written informed consent form.
Author’s contribution
CD: Conception and design of study, drafting of manuscript.
RML: Acquisition of data, critical revision of manuscript for intellectual input.
AN: Analysis and acquisition of data.
MK: Critical revision of manuscript for important intellectual contect.
SD: Conception of the research idea.
ALL AUHTORS: Approval of the final version of the manuscript to be published.
Author details
Chellat Djalila1,2, Rezgoun Mohamed Larbi1,2, Mcelreavey Kenneth3, Abadi Norreddine2, Satta Dalila1,2
- Laboratory of Molecular and Cellular Biology, University Frères Mentouri Constantine 1, Constantine, Algeria
- Laboratory of Biology and Molecular Genetics, Hospital Center University Ben-Badis, University Salah Boubnider Constantine 3, Constantine, Algeria
- Department of Human Developmental Genetics, Pasteur Institute, Paris, France.
References
- Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000Res. 2019;8:F1000 Faculty Rev-670. https://doi.org/10.12688/f1000research.17076.1
- Luo H, Li H, Yao N, Hu L, He T. Association between 3801T>C polymorphism of CYP1A1 and idiopathic male infertility risk: a systematic review and meta-analysis. PLoS One. 2014;9(1):e86649. https://doi.org/10.1371/journal.pone.0086649
- O'Flynn O'Brien KL, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;93(7):1–12. https://doi.org/10.1016/j.fertnstert.2009.10.045
- Jurewicz J, Radwan M, Sobala W, Radwan P, Jakubowski L, Wielgomas B, et al. Exposure to widespread environmental endocrine disrupting chemicals and human sperm sex ratio. Environ Pollut. 2016;213:732–40. https://doi.org/10.1016/j.envpol.2016.02.008
- McManus ME, Burgess WM, Veronese ME, Huggett A, Quattrochi LC, Tukey RH. Metabolism of 2-acetylaminofluorene and benzo(a)pyrene and activation of food-derived heterocyclic amine mutagens by human cytochromes P-450. Cancer Res. 1990;50(43):3367–76.
- Hildebrand CE, Gonzalez FJ, McBride OW, Nebert DW. Assignment of the human 2,3,7,8-tetrachlorodibenzo-p dioxin-inducible cytochrome P1-450 gene to chromosome 15. Nucleic Acids Res. 1985;13(2):2009–16. https://doi.org/10.1093/nar/13.6.2009
- Crofts F, Taioli E, Trachman J, Cosma GN, Currie D, Toniolo P, et al. Functional significance of different human CYP1A1 genotypes. Carcinogenesis. 1994;15(9):2961–3. https://doi.org/10.1093/carcin/15.12.2961
- Petersen DD, McKinney CE, Ikeya K, Smith HH, Bale AE, McBride OW, et al. Human CYP1A1 gene: co-segregation of the enzyme inductibility phenotype and an RFLP. Am J Hum Genet. 1991;48(7):720–5.
- Vani GT, Mukesh N, Siva Prasad B, Rama Devi P, Hema Prasad M, Usha Rani P, et al. Association of CYP1A1*2A polymorphism with male infertility in Indian population. Clin. Chim Acta. 2009;410:43–7. https://doi.org/10.1016/j.cca.2009.09.019
- Franasiak JM, Barnett R, Molinaro TA, Gabriele D, Gartmond TD, Treff NR, et al. CYP1A1 3801T>C polymorphism implicated in altered xenobiotic metabolism is not associated with variations in sperm production and function as measured by total motile sperm and fertilization rates with intracytoplasmic sperm injection. Fertil Steril. 2016;106(2):481–6. https://doi.org/10.1016/j.fertnstert.2016.04.003
- Aydos SE, Taspinar M, Sunguroglu A, Aydos K. Association of CYP1A1 and glutathione S-transferase polymorphisms with male factor infertility. Fertil Steril. 2009;92(2):541–7. https://doi.org/10.1016/j.fertnstert.2008.07.017
- Peng L, Wang G, Jiao H, Li Y, Dang J. Relationship between CYP1A1 rs4646903 gene polymorphism and male infertility in Ningxia. J Ningxia Med Univ. 2012;34(3):208–10.
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45. https://doi.org/10.1093/humupd/dmp048
- Gaspari L, Chang SS, Santella RM, Garte S, Pedotti P, Taioli E. Polycyclic aromatic hydrocarbon-DNA adducts in human sperm as a marker of DNA damage and infertility. Mutat Res. 2003;535(2):155–60. https://doi.org/10.1016/S1383-5718(02)00297-8
- Salehi Z, Gholizadeh L, Vaziri H, Madani AH. Analysis of GSTM1, GSTT1, and CYP1A1 in idiopathic male infertility. Reprod Sci. 2012;19(1):81–5. https://doi.org/10.1177/1933719111413302
- Yarosh SL, Kokhtenko EV, Starodubova NI, Churnosov MI, Polonikov AV. Smoking status modifies the relation between CYP1A1*2C gene polymorphism and idiopathic male infertility: the importance of gene- environment interaction analysis for genetic studies of the disease. Reprod Sci. 2013;20(11):1302–07. https://doi.org/10.1177/1933719113483013
- Lu N, Wu B, Xia Y, Wang W, Gu A, Liang J, et al. Polymorphisms in CYP1A1 gene are associated with male infertility in a Chinese population. Int J Androl 2008;31(5):527–33. https://doi.org/10.1111/j.1365-2605.2007.00804.x
- Wang T, Hu T, Zhen J, Zhang L, Zhang Z. Association of MTHFR, NFKB1, NFKBIA, DAZL and CYP1A1 gene polymorphisms with risk of idiopathic male infertility in a Han Chinese population. Int J Clin Exp Pathol. 2017;10(7):7640–49.
- Ramgir SS, Sekar N, Jindam D, Abilash VG. Association of CYP1A1*2A polymorphism with idiopathic non-obstructive azoospermia in a south Indian cohort. Int J Fertil Steril. 2017;11(3):142-7.
- Fang J, Wang S, Wang H, Zhang S, Su S, Song Z, et al. The cytochrome P4501A1 gene polymorphisms and idiopathic male infertility risk: a meta-analysis. Gene. 2014;10;535(2):93–6. https://doi.org/10.1016/j.gene.2013.11.011
- Nejati M, Karimian M. The rs4646903 gene transition and idiopathic male infertility: a systematic literature review and meta-analysis. Biosci Biotech Res Comm. 2016;9(4):718–24. https://doi.org/10.21786/bbrc/9.4/20
- Cao D, Ren Z, Lu D, Liu L, Xu P, Zhang Q, et al. Association between CYP1A1 rs4646903 T > C genetic variations and male infertility risk: a meta-analysis. Medicine (Baltimore). 2019;98(31):e16543. https://doi.org/10.1097/MD.0000000000016543
- Georgellis A, Toppari J, Veromaa T, Rydstrom J, Parvinen M. Inhibition of meiotic divisions of rat spermatocytes in vitro by polycyclic aromatic hydrocarbons. Mutat Res. 1990;231(2):125–35. https://doi.org/10.1016/0027-5107(90)90019-Z
