Original Article
VOLUME: 38 | ISSUE: 2 | Jun 20, 2022 | PAGE: (88 - 93) | DOI: 10.51441/BioMedica/5-674
Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study
Authors: Sadia Majeed , Usman Aslam , Sehrish Zaffar , Sadia Chiragh
Article Info
Authors
Sadia Majeed
Department of Pharmacology, Continental Medical College, Lahore, Pakistan.
Usman Aslam
Department of Pharmacology, Central Park Medical College, Lahore, Pakistan.
Sehrish Zaffar
Assistant Professor, Department of Pharmacology, CMH Lahore Medical College and Institute of Dentistry (NUMS), Lahore, Pakistan
Sadia Chiragh
Head, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan.
Publication History
Received: February 14, 2022
Revised: May 16, 2022
Accepted: June 06, 2022
Published: June 20, 2022
Abstract
Background and Objective: Hepatotoxicity induced by antituberculosis drugs is quite common and often linked with oxidative stress. Ajwa dates are rich in antioxidants and flavonoids; therefore, these may be protective against the oxidative stress to liver. This study was designed to determine the hepatoprotective effects of Ajwa dates on hepatotoxicity induced by antituberculous drugs in an experimental model.
Methods: This experimental study was conducted at the Post Graduate Medical Institute, Lahore, Pakistan. A total of 30 male rabbits were divided into 5 groups, with 6 animals in each group. Group A and B were fed on normal diet. Group C, D, and E were fed on a diet supplemented with whole Ajwa dates, flesh, and seed powder, respectively. Group B, C, D, and E were given isoniazid 50 mg/kg and rifampicin 100 mg/kg orally for 14 days. After the rabbits were sacrificed, hepatotoxic changes were examined histologically in all groups according to standard criteria.
Results: Liver to body weight ratio was higher in disease group (B) as compared to the healthy control group A (p-value = 0.03), Ajwa flesh group D (p-value = 0.02) and Ajwa seed powder group E (p-value = 0.07). Differences between experimental groups were not statistically significant for both liver weight, and liver weight to body weight ratio. On histological examination, degeneration, necrosis, steatosis, triaditis, and fibrosis were seen in the disease group B while no such changes were observed in group C, D, and E.
Conclusion: Ajwa dates (Phoenix dactylifera L.) has a protective role against isoniazid and rifampicin-induced hepatocellular injury and fibrosis.
Keywords: Ajwa dates, anti-tuberculosis drugs, hepatotoxicity, histology, antioxidant
Pubmed Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh. Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study. BioMedica. 2022; 20 (June 2022): 88-93. doi:10.51441/BioMedica/5-674
Web Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh. Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study. https://biomedicapk.com/articles/online_first/674 [Access: July 27, 2024]. doi:10.51441/BioMedica/5-674
AMA (American Medical Association) Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh. Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study. BioMedica. 2022; 20 (June 2022): 88-93. doi:10.51441/BioMedica/5-674
Vancouver/ICMJE Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh. Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study. BioMedica. (2022), [cited July 27, 2024]; 20 (June 2022): 88-93. doi:10.51441/BioMedica/5-674
Harvard Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh (2022) Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study. BioMedica, 20 (June 2022): 88-93. doi:10.51441/BioMedica/5-674
Chicago Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh. "Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study." 20 (2022), 88-93. doi:10.51441/BioMedica/5-674
MLA (The Modern Language Association) Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh. "Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study." 20.June 2022 (2022), 88-93. Print. doi:10.51441/BioMedica/5-674
APA (American Psychological Association) Style
Sadia Majeed, Usman Aslam, Sehrish Zaffar, Sadia Chiragh (2022) Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study. , 20 (June 2022), 88-93. doi:10.51441/BioMedica/5-674
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 38(2):88-93
ORIGINAL ARTICLE
Ajwa date (Phoenix dactylifera L.) is hepatoprotective against toxicity by antituberculosis drugs - an experimental study
Sadia Majeed1, Usman Aslam2, Sehrish Zaffar3*, Sadia Chiragh4
Received: 14 February 2022 Revised date: 16 May 2022 Accepted: 06 June 2022
Correspondence to: Sehrish Zaffar
*Assistant Professor, Department of Pharmacology, CMH Lahore Medical College and Institute of Dentistry (NUMS), Lahore, Pakistan.
Email: sehrish.zaffar@gmail.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Hepatotoxicity induced by antituberculosis drugs is quite common and often linked with oxidative stress. Ajwa dates are rich in antioxidants and flavonoids; therefore, these may be protective against the oxidative stress to liver. This study was designed to determine the hepatoprotective effects of Ajwa dates on hepatotoxicity induced by antituberculous drugs in an experimental model.
Methods:
This experimental study was conducted at the Post Graduate Medical Institute, Lahore, Pakistan. A total of 30 male rabbits were divided into 5 groups, with 6 animals in each group. Group A and B were fed on normal diet. Group C, D, and E were fed on a diet supplemented with whole Ajwa dates, flesh, and seed powder, respectively. Group B, C, D, and E were given isoniazid 50 mg/kg and rifampicin 100 mg/kg orally for 14 days. After the rabbits were sacrificed, hepatotoxic changes were examined histologically in all groups according to standard criteria.
Results:
Liver to body weight ratio was higher in disease group (B) as compared to the healthy control group A (p-value = 0.03), Ajwa flesh group D (p-value = 0.02) and Ajwa seed powder group E (p-value = 0.07). Differences between experimental groups were not statistically significant for both liver weight, and liver weight to body weight ratio. On histological examination, degeneration, necrosis, steatosis, triaditis, and fibrosis were seen in the disease group B while no such changes were observed in group C, D, and E.
Conclusion:
Ajwa dates (Phoenix dactylifera L.) has a protective role against isoniazid and rifampicin-induced hepatocellular injury and fibrosis.
Keywords:
Ajwa dates, anti-tuberculosis drugs, hepatotoxicity, histology, antioxidant.
Introduction
Tuberculosis (TB) is one of the most common infectious diseases, affecting millions throughout the world per annum. Drugs used for the treatment of TB include isoniazid, rifampicin, ethambutol, and pyrazinamide. These drugs are used in combination for maximum efficacy and prevention of the emergence of resistant strains. Although effective, all of these drugs are notorious for causing serious adverse effects such as hepatotoxicity and neuropathy.1
The reported incidence of hepatic damage caused by co-administration of isoniazid and rifampicin is 2.0%.2 drug-induced liver injury (DILI) can manifest as an acute hepatocellular insult, cholestatic injury, or mixed liver injury. Among DILI, hepatocellular and cholestatic forms of liver injury are the most prevalent.3 Metabolic oxidation of acetyl hydrazine leads to the formation of oxidative species that bind covalently to proteins and cause hepatic damage and shows a strong correlation between oxidative stress and hepatic insult.4
Isoniazid acts by inhibiting the synthesis of mycolic acid in Mycobacterium tuberculosis. It is metabolized in the liver by acetylation to acetyl-isoniazid which is further metabolized to mono-acetyl hydrazine, diacetyl hydrazine, and a few other metabolites. Free radicals produced from the metabolism of mono-acetyl hydrazine and covalent bonding of acetyl hydrazine to macromolecules of the liver cause hepatocytes injury.5
Rifampicin is used in combination with isoniazid for TB treatment. It hinders the growth of M. tuberculosis via inhibition of the mycobacterial DNA-dependent RNA polymerase.6 Rifampicin induces the function of hepatic enzymes. It also induces oxidative stress and impairs the antioxidant defense in liver cells.7 Pyrazinamide is metabolized to pyrazinoic acid, which is hepatotoxic. It also alters nicotinamide acetyl dehydrogenase levels in the liver, leading to the production of free radicals that cause liver injury.8
Ajwa date (Phoenix dactylifera L.) is among one of the most ancient fruit crops. It is commonly grown in the Middle East, North Africa, and the Arabian Peninsula. The flesh and seeds of Ajwa dates are rich in alkaloids, protein, carbohydrates, fatty acids, vitamins, minerals, polyphenolic compounds, flavonoids, and tannins.9,10 Ajwa dates are also known to possess strong antioxidant activity due to their high content of polyphenols and flavonoids.11 Ajwa dates have shown the protective effect of seeds against carbon tetrachloride (CCl4) and paracetamol-induced liver lesions.12,13
This study is therefore designed to observe the antioxidant and protective effects of Ajwa dates on hepatotoxicity induced by anti-TB drugs.
Methods
This experimental study was conducted at the Post Graduate Medical Institute, Lahore, Pakistan from November 2016 to March 2017 after getting approval by the Institutional Ethical Committee. A sample size of 30 was calculated by applying 90% power of study and 5% level of significance.14 Healthy adult albino male rabbits, approximately 4 months old, weighing 1.2-1.5 kg, were purchased from the local market. The animals were categorized into five groups, with six animals in each group. They were kept for 1 week for acclimatization, under standard laboratory conditions, before starting the study.15
The rabbits were fed recommended diet and water. Diet pellets were prepared with split chickpeas, dry fodder, jawar, and plain flour.15 All ingredients were mixed with water and pellets were formed. For whole fruit supplementation, flesh and seeds of Ajwa dates were mixed with rabbit diet.
Thirty rabbits were divided into five groups. Group A and B were fed on a normal diet. Group C, D and E were fed on diet supplemented with whole Ajwa dates, flesh and seed powder, respectively (one date/100 g). Hepatotoxicity was induced in Group B, C, D, and E by the administration of single daily dose of isoniazid 50 mg/kg and rifampicin 100 mg/kg orally for 14 days.15 Both drugs were obtained from Schazoo Zaka Pharmaceuticals, Pakistan Ltd.
For histological examination of the liver, rabbits were sacrificed after 14 days. Liver was dissected out and weighed. The liver to body weight ratio was estimated. Tissue sections from the liver were fixed in 10% formalin. After processing, approximately 3-5 mm thick sections of tissue were cut by rotary microtome and stained with hematoxylin and eosin (H&E). Pathological changes were observed under high power field. Whole slide was thoroughly studied for presence or absence of degeneration, steatosis, necrosis, fibrosis, and degeneration.16
Statistical analysis
Statistical Package for the Social Sciences version. 20 was used for analyzing the data. One way analysis of variance was used for comparison of liver weight and liver to body weight ratio. To find the difference in mean between the groups, post hoc Tukey’s test was applied. The significance of the difference in time in all groups was calculated by a paired t-test. Fischer’s exact test was applied for comparison of qualitative parameters (degeneration, necrosis, fibrosis, steatosis, and triaditis). The p-value < 0.05 was considered to be significant.
Results
Mean liver weights of experimental groups (C, D, and E) were lower than both groups A and B. The difference of group D only was significant from group A (p-value = 0.03) and B (p-value = 0.02). Liver to body weight ratio was significantly high in group B, in comparison to group A (p-value = 0.03), group D (p-value = 0.02) and group E (p-value = 0.07). Differences between experimental groups C, D, and E were not statistically significant for hepatic weight, alone, or hepatic weight to body weight ratio. Table 1 shows the mean and standard deviation of hepatic weight and the hepatic weight to body weight ratio.
Administration of anti-TB drugs for 14 days to group B resulted in degeneration, necrosis, steatosis, triaditis, and fibrosis as evident on histological examination of rabbit liver. Concurrent administration of Ajwa date whole to group C, flesh to group D and seed powder to group E prevented the histological damage to liver and caused a significant reduction in all histopathological parameters (Table 2).
Hepatocytes were arranged in cords in relation to central vein and sinusoids as seen in histological sections of liver from the control group (Figure 1a). General architecture of hepatic tissue was lost in liver sections from rabbits treated with anti-TB drugs (group B) and showed steatosis with vacuolation (Figure 1b). Changes in cytoplasmic content and pyknotic nuclei confirming degenerative changes were observed in group B (Figure 1c and d). Furthermore, liver sections of anti-TB drugs treated group showed marked bridging fibrosis (Figure 1e).
The groups treated with Ajwa date whole, flesh and seed powder (group C, D, and E, respectively), showed marked ameliorations in hepatic lesions, with less obvious inflammatory and degenerative changes. Steatosis and fibrosis were markedly reduced along with almost normal hepatic strands with lesser dilatation of central veins and sinusoids. Although some vacuolated and degenerative cells were also seen but they were few in number (Figure 2).
Table 1. Effect of Ajwa date on hepatic weight and hepatic weight to body weight ratio (n = 6).
| Groups | Hepatic weight (g) | Hepatic to body weight ratio |
|---|---|---|
| Group A (normal control) | 38.5 ± 1.2 | 0.029 ± 0.002 |
| Group B (hepatotoxic control) | 38.6 ± 1.1 | 0.033 ± 0.002≠ |
| Group C (Ajwa date whole) | 371 ± 0.7 | 0.031 ± 0.002 |
| Group D (Ajwa date flesh) | 36.6 ± 1.1 | 0.029 ± 0.001* |
| Group E (Ajwa date seed) | 373 ± 1.0 | 0.029 ± 0.001* |
| p-value | 0.009 | 0.001 |
≠p-value < 0.05 versus group A,
*p-value < 0.05 versus group B.
Table 2. Histological changes after administration of Ajwa date following hepatoxicity induced by antituberculosis drugs in Groups B-E (n = 6).
| Histological parameters | Group An (%) | Group Bn (%) | Group Cn (%) | Group Dn (%) | Group En (%) | p-value |
|---|---|---|---|---|---|---|
| Degeneration | 0 (0.0) | 6 (100.0) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 0.004 |
| Necrosis | 0 (0.0) | 5 (83.3) | 1 (16.7) | 1 (16.7) | 2 (33.3) | 0.023 |
| Steatosis | 0 (0.0) | 6 (100.0) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 0.004 |
| Fibrosis | 0 (0.0) | 6 (100.0) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 0.004 |
| Triaditis | 0 (0.0) | 5 (83.3.0) | 0 (0.0) | 2 (33.3) | 2 (33.3) | 0.010 |
| Regeneration | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
Discussion
The therapeutic use of isoniazid and rifampicin, for the treatment of TB, is a well-known cause of liver injury.17 This study was designed to evaluate the protective role of Ajwa date in anti-TB drugs induced hepatotoxicity in the animal model. The current study reports hepatocytes degeneration, necrosis, steatosis, triaditis, and fibrosis of the rabbit’s liver after administration of isoniazid and rifampicin. However, the concurrent administration of Ajwa date whole, its flesh, and seed powder prevented the histological damage to the liver and caused a significant reduction in all histopathological changes.
According to the previous studies, the measurement of single organ weight is more reliable, as compared to whole-body weight.18 Since body weight decreases in hepatotoxic studies, therefore, liver-to-body weight ratio was also estimated.
In this study, hepatic weight and hepatic to whole body weight ratio were decreased in Ajwa treated groups in comparison to the diseased group, but the difference between the treated groups was statistically insignificant. These results are similar to a Chinese study in which the effect of a herb was observed on DILI in rats and no significant difference was observed in hepatic weight and hepatic to body weight ratio.19 However, hepatic to body weight ratio was observed to decrease significantly, with the administration of date seed powder, against CCl4-induced hepatotoxicity.12
It was observed that administration of anti-TB drugs resulted in degeneration, necrosis, steatosis, triaditis, and fibrosis of the rabbit’s liver. However, the concurrent administration of Ajwa date whole, its flesh, and seed powder preserved the histological structure of the liver by significantly reducing these changes. A similar study was done by Kosasih and colleagues.20 to evaluate the hepatoprotective and antioxidant potential of Ajwa fruit powder. The results were by the current study. The authors deduced that the presence of flavonoids and vitamin C, in Ajwa date fruit powder, was the major reason for the prevention of hepatocellular injury. Similarly, histopathological changes were seen in another study, including portal inflammation, necrosis, fatty changes, and ballooning degeneration of hepatocytes pretreated with isoniazid and rifampicin. These changes were reduced markedly when co-treated with pyrrolidine dithiocarbamate.21 Similarly, work done by Abdelaziz and Ali12 demonstrated the hepatoprotective effect of Hayani date seed powder against CCl4-induced hepatotoxicity by decreasing degeneration, vacuolization, and fibrosis. Antioxidant activity seems to be the most probable mechanism of hepatoprotective effect of Ajwa dates. Lipid peroxidation is mainly responsible for inflammatory changes and damage to liver tissue. Ajwa date causes a decrease in lipid peroxidation of hepatocellular membrane and increases the process of repair possibly due to the presence of antioxidants and vitamins, such as vitamin E.11 Multiple studies have documented an increase in antioxidant activity as a result of administration of date fruit and seed powders. Increased levels of superoxide dismutase and glutathione-S-transferase, reported in these studies, have been linked to the enhanced free radical scavenging capacity that counters oxidative stress and protects the cellular injury.12-14
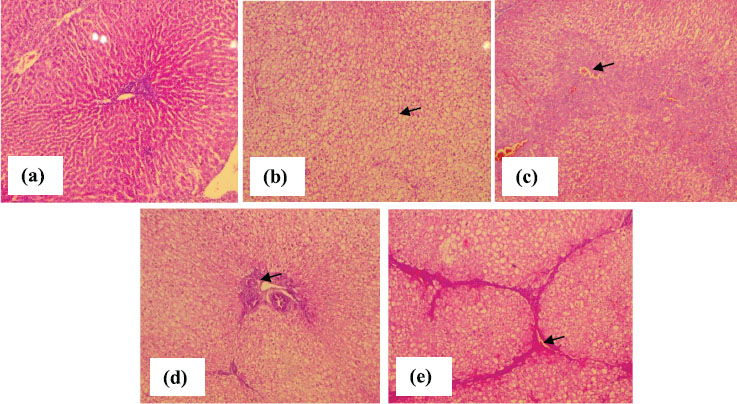
Figure 1. Photomicrographs of liver tissue showing (a) normal architecture arranged as sheets of hepatocytes (b) steatotic changes (c) triaditis along with degenerative and necrotic changes (d) feathery degeneration (e) bridging fibrosis (H&E, ×40).
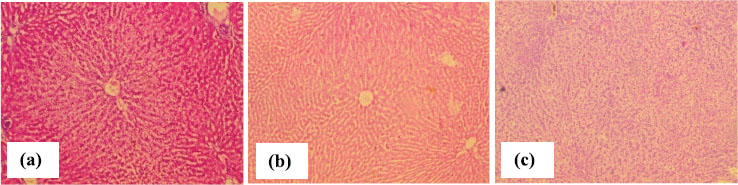
Figure 2. Photomicrographs of liver tissue showing (a) cords of hepatocytes and almost normal architecture in anti-TB drugs and whole date treated group (b) cords of hepatocytes around central vein in antituberculous drugs and Ajwa date flesh group (c) less hydropic and necrotic changes in anti-TB drugs and Ajwa date seed powder group (H&E, ×40).
The hepatoprotective role of Ajwa date may also be related to its inhibitory effect on inflammation. Inflammatory changes have a profound impact on causing drug-induced hepatitis, as evident by the abundance of arachidonic acid derivatives, commonly seen during the inflammatory process. Ajwa date has been shown to possess anti-inflammatory activity.22 Therefore, hepatoprotection can be reliably linked to anti-inflammatory properties as well.
Conclusion
It is concluded that Ajwa date whole, flesh, and seed powder can protect the liver against isoniazid and rifampicin-induced damage. Whole dates are more beneficial in preventing hepatic cellular injury as compared to flesh or seed powder alone.
Limitations of the study
This study has few limitations. First, only one variety of dates was used. Second, biochemical parameters could have been added to augment the histological parameters of hepatotoxicity.
Acknowledgment
The authors would like to acknowledge the staff and doctors of the Postgraduate Medical Institute (PGMI), Lahore, Pakistan for their logistic and technical support during the execution of the study.
List of Abbreviations
| CCl4 | Carbon tetrachloride |
| DILI | Drug-induced liver injury |
| H&E | Hematoxylin and eosin |
| NADH | Nicotinamide acetyl dehydrogenase |
| TB | Tuberculosis |
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose.
Ethical approval
The Institutional Ethical Review Board of Postgraduate Medical Institute (PGMI), Lahore, Pakistan approved the study with ethical approval number 00-08-5-2016 dated 15.04.2016.
Authors’ contribution
SM: Conception and design of the study, data collection and drafting of the manuscript.
UA: Acquisition, analysis and interpretation of data.
SZ: Analysis of data, important intellectual input and drafting of the manuscript.
SC: Conception and design of the study, Important intellectual input.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ Details
Sadia Majeed1, Usman Aslam2, Sehrish Zaffar3, Sadia Chiragh4
- Department of Pharmacology, Continental Medical College, Lahore, Pakistan
- Department of Pharmacology, Central Park Medical College, Lahore, Pakistan
- Assistant Professor, Department of Pharmacology, CMH Lahore Medical College and Institute of Dentistry (NUMS), Lahore, Pakistan
- Head, Department of Pharmacology, Al-Aleem Medical College, Lahore, Pakistan
References
- Sharma A, De Rosa M, Singla N, Singh G, Barnwal RP, Pandey A. Tuberculosis: an overview of the immunogenic response, disease progression, and medicinal chemistry efforts in the last decade toward the development of potential drugs for extensively drug-resistant tuberculosis strains. J Med Chem. 2021;64(8):4359–95. https://doi.org/10.1021/acs.jmedchem.0c01833
- Gill MK, Patyar RR, Patyar S. Antitubercular drug induced hepatotoxicity: a review. Eur J Mol Clin Med. 2020;7(7):2840–47. Available from: https://ejmcm.com/article_4990_eef4948246ee32ef2143d39a2d682607.pdf
- Hayase N, Doi K, Hiruma T, Inokuchi R, Hamasaki Y, Noiri E, et al. Damage-associated molecular patterns in intensive care unit patients with acute liver injuries: A prospective cohort study. Medicine. 2018;97(41):e12780. https://doi.org/10.1097/md.0000000000012780
- Yew WW, Chang KC, Chan DP. Oxidative stress and first-line antituberculosis drug-induced hepatotoxicity. Antimicrob Agents Chemother. 2018;2(8):e02637–17. https://doi.org/10.1128/aac.02637-17
- Lei S, Gu R, Ma X. Clinical perspectives of isoniazid-induced liver injury. Liver Res. 2021;5(2):45–52. https://doi.org/10.1016/j.livres.2021.02.001
- Idowu T, Arthur G, Zhanel GG, Schweizer F. Heterodimeric rifampicin–tobramycin conjugates break intrinsic resistance of Pseudomonas aeruginosa to doxycycline and chloramphenicol in vitro and in a Galleria mellonella in vivo model. Eur J Med Chem. 2019;174:16–32. https://doi.org/10.1016/j.ejmech.2019.04.034
- Su Q, Kuang W, Hao W, Liang J, Wu L, Tang C, et al. Antituberculosis drugs (rifampicin and isoniazid) induce liver injury by regulating NLRP3 inflammasomes. Mediators Inflamm. 2021;2021:8086253. https://doi.org/10.1155/2021/8086253
- Lamont EA, Dillon NA, Baughn AD. The bewildering antitubercular action of pyrazinamide. Microbiol Mol Biol Rev. 2020;84(2):e00070–19. https://doi.org/10.1128/mmbr.00070-19
- El-Far AH, Oyinloye BE, Sepehrimanesh M, Allah MA, Abu-Reidah I, Shaheen HM, et al. Date palm (Phoenix dactylifera): novel findings and future directions for food and drug discovery. Curr Drug Discov Technol. 2019;16(1):2–10. https://doi.org/10.2174/1570163815666180320111937
- Qadir A, Shakeel F, Ali A, Faiyazuddin M. Phytotherapeutic potential and pharmaceutical impact of Phoenix dactylifera (date palm): current research and future prospects. J Food Sci Technol. 2020;57(4):1191–204. https://doi.org/10.1007/s13197-019-04096-8
- Al-Shwyeh HA. Date palm (Phoenix dactylifera L.) fruit as potential antioxidant and antimicrobial agents. J Pharm Bioallied Sci. 2019;11(1):1–11. https://doi.org/10.4103/jpbs.jpbs_168_18
- Abdelaziz DH, Ali SA. The protective effect of Phoenix dactylifera L. seeds against CCl4-induced hepatotoxicity in rats. J Ethnopharmacol. 2014;155(1):736–43. https://doi.org/10.1016/j.jep.2014.06.026
- Bouhlali ED, Derouich M, Hmidani A, Bourkhis B, Khouya T, Filali-Zegzouti Y, et al. Protective effect of Phoenix dactylifera L. seeds against paracetamol-induced hepatotoxicity in rats: a comparison with vitamin C. Sci World J. 2021;2021:6618273. https://doi.org/10.1155/2021/6618273.
- Bakr Abdu S. The protective role of ajwa date against the hepatotoxicity induced by ochratoxin A. Egy J Nat Toxinsn. 2011;8(1):1–15. Available from: https://eujournal.org/index.php/esj/article/view/2957/2913
- Majeed S, Aslam U, Khan MU, Inam A, Chiragh S. Phoenix dactylifera (ajwa date) whole fruit, flesh and powdered seed prevents anti-tuberculous drug induced hepatotoxicity in rabbits. Proceedings. 2021;35(3):58–63. Available from: https://proceedings-szmc.org.pk/index.php/szmc/article/view/146
- Yusrini DY, Adnan J, Amaliah N, Ramli N, Sartini S, Salang MS, et al. Roselle (Hibiscus sabdariffa L.) calyx water extract ameliorates isoniazid and rifampicin induced liver and renal injuries in rats. J Herbmed Pharmacol. 2021;10(3):296–303. https://doi.org/10.34172/jhp.2021.34
- Biswas A, Santra S, Bishnu D, Dhali GK, Chowdhury A, Santra A. Isoniazid and Rifampicin produce hepatic fibrosis through an oxidative stress-dependent mechanism. Int J Hepatol. 2020;2020:e6987295. https://doi.org/10.1155/2020/6987295
- Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32(4):448–66. https://doi.org/10.1080/01926230490465874
- Chen Y, Mo Q, Xie B, Ma B, Zang X, Zhou G, et al. Hepatoprotective activity of Yigan Mingmu oral liquid against isoniazid/rifampicin-induced liver injuries in rats. Chin Med. 2018;9(04):165–78. Available from: https://www.scirp.org/pdf/CM_2018120416341358.pdf
- Kosasih E, Chiuman L, Lister IN, Fachrial E. Hepatoprotective effect of citrus sinensis peel extract against isoniazid and rifampicin-induced liver injury in wistar rats. Trad Med J. 2019;24(3):197–203. https://doi.org/10.22146/mot.45762
- He X, Song Y, Wang L, Xu J. Protective effect of pyrrolidine dithiocarbamate on isoniazid/rifampicininduced liver injury in rats. Mol Med Rep. 2020;21(1):463–9. https://doi.org/10.3892/mmr.2019.10817
- El-Abed H, Chakroun M, Abdelkafi-Koubaa Z, Drira N, Marrakchi N, Mejdoub H, et al. Antioxidant, anti-inflammatory, and antitumoral effects of aqueous ethanolic extract from Phoenix dactylifera L. parthenocarpic dates. BioMed Res Int. 2018;2018:e1542602. https://dx.doi.org/10.1155%2F2018%2F1542602
