Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 39(4):150-155
ORIGINAL ARTICLE
Formoterol induces muscle regeneration through myogenic regulatory factor MyoD in rat model of statin-induced myopathy
Abdullah Qamar1*, Shabnam Hamid2, Fareeha Mushtaq3, Muhammad Saad Abdullah4, Faiza Umbreen5, Rabya Khalid6
Received: 08 July 2023 Revised date: 31 Oct 2023 Accepted: 11 Nov 2023
Correspondence to: Abdullah Qamar
*Associate Professor, Department of Anatomy, Army Medical College, Rawalpindi, Pakistan.
Email: drabdullahqamar@gmail.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Statins can induce myopathy; however, the impact on muscle regeneration remains unclear. β2-agonists may mitigate statin myotoxicity. This study investigated the effects of simvastatin and formoterol on skeletal muscle regeneration and injury using a rat model.
Methods:
Adult Sprague-Dawley rats (n = 90) were divided into three groups: control (A), simvastatin treatment (B), and simvastatin plus formoterol treatment (C). The rats were kept at room temperature for 3 months where an animal house diet was available ad libitum. Simvastatin was administered orally once a day, by oral gavage for 12 weeks; formoterol was concurrently administered to one group. Extensor digitorum longus muscles were analyzed for the myogenic differentiation factor (MyoD) by immunohistochemistry. Serum lactate dehydrogenase (LDH) levels were quantified as a marker of muscle damage.
Results:
MyoD-positive myonuclei were significantly increased in the C group (simvastatin plus formoterol group) compared to the A and B Groups (control and simvastatin-only groups) (p < 0.05). Serum LDH levels were significantly lower in group C than in groups A and B (p < 0.05).
Conclusion:
Formoterol alleviates statin-associated muscle injury in a rat model as evidenced by increased MyoD expression and decreased LDH levels. The activation of dormant satellite cells and increased expression of MyoD indicate the potential of formoterol to promote muscle regeneration.
Keywords:
Statin myotoxicity, β2-agonists, formoterol, MyoD, lactate dehydrogenase.
Introduction
Statins are cholesterol-lowering agents extensively prescribed as first-line agents for the treatment of hypercholesterolemia and prevention of coronary heart disease1. Statin-induced myopathy is the leading cause of statin intolerance and withdrawal2. Statin-induced myopathies deserve special importance because they are often overlooked, resulting in misdiagnosis and improper care and treatment. Patients report to clinicians with symptoms of fatigue, muscle weakness, pain and tenderness, night time cramping, and worsening of these complaints with enhanced physical activities3. Many theories have been suggested to elucidate statin-induced myotoxicity. These include the reduction of mevalonate pathway products, including metabolites of isoprenoids, mitochondrial dysfunction, and the induction of apoptosis. Gaining insights into the molecular, cellular, and environmental factors governing skeletal muscle repair may help in the development of effective therapies for a variety of skeletal muscle diseases and injuries. Skeletal muscle satellite cells (SCs) are dormant mononucleated myogenic cells responsible for postnatal muscle growth and regeneration in response to injury4,5. They are activated from this dormant state to accomplish their roles in the maintenance and repair of adult skeletal muscle in postnatal development and repair of skeletal muscle4,6. In response to muscle fiber injury, quiescent SCs become active and express myogenic regulatory factors (MRFs). MRFs regulate gene expression and myoblast proliferation and differentiation7. Myogenic differentiation factor (MyoD) is an important MRFs in myogenic determination8. It is expressed in proliferating myoblasts and continues to be expressed even after muscle determination has occurred. MyoD stimulates skeletal muscle cell division and replication and enhances the incorporation of SCs into existing myofibers. It has been seen that the structural and functional recovery of myotoxic injury can be accelerated by the stimulation of β-adrenoceptors in skeletal muscles9. Thus, the basis of the effectiveness of β-agonists in skeletal myopathy is their ability to stimulate muscle protein synthesis and diminish muscle protein degradation10. Newly established β2 agonists, such as formoterol, have more significant and potent protective effects on skeletal muscle histology than older generations of β211.
Serum lactate dehydrogenase (LDH) level is an indicator of muscle tissue damage. LDH levels help to ascertain the progress of muscle repair and regeneration. This biochemical marker is also used to assess the effectiveness of various therapeutic modalities in muscular diseases12,13. The extensor digitorum longus (EDL) muscle is an optimal model for analyzing skeletal muscle histology and regeneration due to its mixed fiber-type composition, superficial anatomy that allows direct access without disturbing other structures, and established utility in prior muscle studies.
This study aimed to investigate whether the β2-agonist formoterol could mitigate statin-induced muscle injury in a rat model by evaluating its effects on MyoD expression and skeletal muscle regeneration, as assessed by immunohistochemical analysis of MyoD+ myonuclei and serum LDH levels. Gaining insights into the comprehensive understanding of how β2-agonists affect MRFs within myofibers will help us establish their specific role in muscle development, growth, and regeneration. This in turn may help in the development of effective treatment modalities for a variety of skeletal muscle diseases in humans.
Methods
This study was a laboratory-based experimental randomized controlled trial. This research was carried out at the Anatomy Department of the Army Medical College, Rawalpindi, in collaboration with the National Institute of Health (NIH) Islamabad and the Armed Forces Institute of Pathology (AFIP) Rawalpindi. Ninety adult male Sprague-Dawley rats with an average age of approximately 70-80 days and weighing 250 ± 50 g were procured from NIH Islamabad. The rats were kept at a room temperature of 18°C-26°C in separate cages for 3 months, where a normal animal house diet was available ad libitum. The animals were randomly divided into 3 groups, each with 30 rats, numbered separately from 1 to 30. Control Group A (Group A) did not receive any medication. The rats in Group B were administered simvastatin dissolved in distilled water once daily by oral gavage (60 mg/kg/day)14,15 for 12 weeks. Group C received formoterol by oral gavage (3 µg/kg/day)15 dissolved in distilled water once a day for 12 weeks in addition to simvastatin. A 5 ml blood sample was collected from the heart of each animal directly into a plain tube to quantitatively measure the levels of serum LDH before animal sacrifice. The collected samples were labeled appropriately based on the respective experimental groups.
After the experimental period, the animals were sacrificed and the EDL muscle was dissected along with its tendon. A 0.5 cm thick transverse section was obtained from the mid-belly region of the muscle for processing. The tissues were processed to form paraffin-embedded blocks. Standard tissue processing protocols were followed to fix, dehydrate, clear, infiltrate, embed, section, and mount the EDL muscle pieces to create paraffin-embedded blocks for further analysis. Immunohistochemical staining was performed on the slides using a mouse anti-human MyoD antibody for MyoD expression (Santa Cruz Biotechnology, Catalogue number:SC-32758). One cross section was selected from each specimen for immunohistochemical analysis. An indirect immunohistochemical technique through avidin-biotin complex (ABC) method was used for MyoD staining. Briefly, antigen retrieval was performed by heating the tissue sections in citrate buffer at pH 6.0 for 10 minutes. Endogenous peroxidase activity was quenched using 3% H2O2 in methanol for 30 minutes. Sections were blocked with 5% normal goat serum for 60 minutes and then incubated with the primary antibody overnight at 4°C. Biotinylated goat anti-mouse IgG secondary antibody (Vector Labs, catalog #BA-9200) was applied for 30 minutes, followed by incubation with ABC reagent (Vector Labs) for 30 minutes. Color development was performed using a diaminobenzidine substrate kit (Vector Laboratories, Burlingame, CA). The sections were counterstained with Mayer’s hematoxylin, dehydrated, and mounted. The interpretation of MyoD positivity was based on the study by Cornelison and Wold16 where MyoD positive myonuclei stained dark brown, while MyoD negative myonuclei lacked staining and appeared blue. The total number of MyoD positive (brown) myonuclei was counted in eight microscopic fields under 40×objective (by ImageJ software which is a Java-based image processing program) and the average number was recorded.
Statistical analysis
Data were analyzed using the IBM SPSS 27. Quantitative variables are expressed as the mean ± SEM. Analysis of variance (ANOVA) followed by post-hoc Tukey’s test was used to determine the differences among the various groups. Statistical significance was set at p < 0.05.
Table 1. Comparison of immunohistochemical expression of MyoD+ nuclei among three groups A, B, and C.
| Parameters | Groups | Mean ± SEM | Statistical significance (ANOVA) followed by post-hoc Tukey's test | ||
|---|---|---|---|---|---|
| Group A/B | Group A/C | Group B/C | |||
| Number of MyoD+ myonuclei | A | 17.23 ± 0.259 | p < 0.001 | p < 0.001 | p < 0.001 |
| B | 22.60 ± 0.334 | ||||
| C | 31.63 ± 0.344 | ||||
Results
Microscopic examination of the EDL muscle of the control group revealed a normal histological structure of the skeletal muscle, evident by uniformly distributed muscle fibers within fascicles and peripherally positioned myonuclei. In the control group A, MyoD+ myonuclei appeared dark brown upon immunohistochemical staining. The mean number of MyoD+ myonuclei in the control group was 17.23 ± 0.259 (Table 1).
The cross-section of the skeletal muscle of group B showed polygonal bundles of skeletal muscle fibers with peripherally located nuclei. The mean number of MyoD+ myonuclei in the skeletal muscle sections of group B (Figure 1) was 22.60 ± 0.334. This difference was statistically significant in control group A (p < 0.001) (Table 1).
Cross section of EDL of group C showed polygonal-shaped bundles of skeletal muscle fibers having peripherally located nuclei. The number of MyoD+ myonuclei was counted in sections of skeletal muscle (Figure 2) and a mean count of 31.63 ± 0.344 was observed. It was significantly higher when compared to groups A and B (p < 0.001) (Table 1).
Serum LDH levels were measured at the conclusion of the 3-month study period. In group A, the mean LDH levels were 158.18 ± 1.457 IU/l while it was 195.84 ± 2.185 IU/l for group B with a significant difference among both groups (p < 0.001). However, in group C, the mean LDH was 171.54 ± 3.350 IU/l which was significantly lower than that of group B but significantly higher than that of control group A (p < 0.001) (Table 2).
Discussion
Skeletal muscle has a remarkable capacity for regeneration, which relies on muscle stem cells known as SCs. Upon muscle injury, SCs are activated to proliferate and fuse to form new muscle fibers for repair17. This process requires coordinated expression of MRFs such as MyoD18.
A key finding of the current study was that treatment with the β2-adrenergic agonist formoterol significantly increased MyoD+ myonuclei in the regenerating muscle, indicating enhanced SC activation and myogenic potential to stimulate muscle repair. Specifically, formoterol administration resulted in greater restoration of muscle architecture by day 90 post-injury compared to untreated injured muscle.
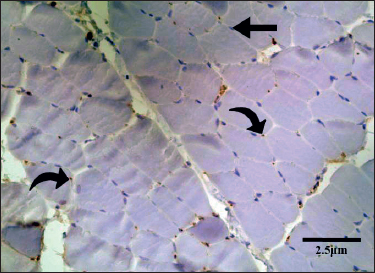
Figure 1. Transverse section of the EDL of a rat from group B. There are scattered MyoD positive satellite nuclei (curved arrow). MyoD-negative myonuclei lacked staining and appeared blue (straight arrows).
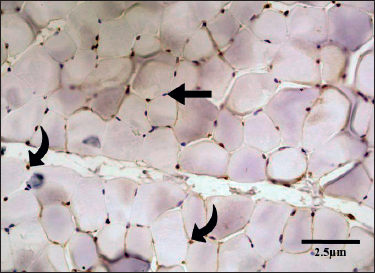
Figure 2. Transverse section of the EDL of a rat in Group C. There were numerous MyoD-positive satellite nuclei (curved arrows). (IHC, 400×). MyoD-negative myonuclei lacked staining and appeared blue (straight arrows).
Table 2. Comparison of LDH levels in three groups.
| Parameters | Groups | Mean ± SEM | Statistical significance (ANOVA) followed by post-hoc Tukey's test | ||
|---|---|---|---|---|---|
| Group A/B | Group A/C | Group B/C | |||
| LDH (IU/l) | A | 158.18 ± 1.457 | p < 0.001 | p < 0.001 | p < 0.001 |
| B | 195.84 ± 2.185 | ||||
| C | 171.54 ± 3.350 | ||||
Previous studies of formoterol have provided insights into the mechanisms underlying its beneficial effects on muscle regeneration. Formoterol acts via β2-adrenergic receptors on muscles, triggering intracellular cyclic adenosine monophosphate-dependent signaling involved in muscle growth19. In rats, formoterol treatment activates anabolic pathways, leading to muscle hypertrophy and strength gain20. Formoterol also inhibits proteolytic pathways and prevents muscle wasting in immobilized rats21.
In our study, formoterol-treated muscles showed increased MyoD+ nuclei and new myofiber formation, indicative of muscle hyperplasia. While we did not quantify the fiber number, prior work showed that formoterol increased myofiber formation in rats following botox-induced muscle paralysis22. MyoD drives myogenic differentiation, implying that formoterol improves regeneration by enhancing MyoD-mediated SC differentiation into mature muscles23.
The analysis of serum LDH levels in the different experimental groups revealed significant variations. In group C, the LDH levels were significantly lower than those in group B, but significantly higher than those in group A (Table 2). These findings suggest that formoterol administration contributes to a reduction in LDH levels by promoting the restoration of skeletal muscle structure in degenerated muscles. Notably, animals exhibiting more severe histomorphological changes displayed higher LDH levels, whereas those showing better restoration of muscle architecture exhibited reduced LDH levels.
Our findings align with the already published literature, indicating that formoterol can stimulate muscle growth and regeneration24. These results shed light on the potential of formoterol as a treatment for muscle diseases. Additional preclinical studies should be conducted to further explore the efficacy of formoterol in treating myopathies. Examining markers such as MyoD and LDH gives us clues into how formoterol may improve muscle repair at the molecular level. Overall, this study adds to the evidence that formoterol could prove useful as a therapy for certain muscle disorders. Further animal model testing is warranted to better understand whether formoterol may benefit patients with different types of muscle pathology.
Conclusion
This study concludes that formoterol improved the regenerative abilities of skeletal muscle cells in a rat model of statin-induced myopathy. The activation of SCs increased MyoD expression and decreased LDH levels suggesting that formoterol may promote muscle regeneration. These results add to our understanding of how muscles can be restored after injury and point to potential therapies for muscle damage and disease in the future.
Limitations of the study
Despite providing valuable insights, this study has several limitations. It relies on animal models and lacks clinical data from human subjects. Although animal studies are informative, the lack of human data makes it difficult to directly apply these findings to real-world medical situations. In addition, the 12-week duration, although reasonable, may not fully capture the long-term impacts of the treatments that could emerge over longer periods. These results support the use of formoterol as a possible therapy for patients with high cholesterol and pulmonary diseases who also have muscle-related morbidities subsequent to the use of statins. Combining formoterol with statins could potentially control asthmatic symptoms and alleviate the myopathic side effects. Further clinical studies in humans are recommended to directly test the effects and safety of formoterol over extended periods of time.
Acknowledgement
The authors wish to acknowledge the management of the Army Medical College, Rawalpindi, Pakistan, for providing the facilities that enabled this study to be conducted. The authors are thankful to the NIH, Islamabad, and AFIP, Rawalpindi, Pakistan, for their collaboration and support with this project. They are grateful to the laboratory technicians and staff who assisted with the tissue processing and immunohistochemistry.
List of Abbreviations
| EDL | Extensor digitorum longus |
| LDH | Lactate dehydrogenase |
| MRF | Myogenic regulatory factor |
| MyoD | Myogenic differentiation factor |
| SC | Satellite cell |
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose.
Ethical approval
This study was approved by the Ethical Review Committee of Army Medical College, Rawalpindi, Pakistan, vide Letter No. ERC ID/375 dated 22 Oct 2021.
Authors’ contributions
AQ: Conception and design of study, acquisition of data, drafting of the manuscript with critical intellectual input.
SH: Design of study, acquisition, and analysis of data, drafting of the manuscript
FM: Acquisition of data, critically intellectual input
MSA: Acquisition of data, drafting of the manuscript
FU: Critical intellectual input, drafting of the manuscript
RK: Analysis of data.
ALL AUTHORS: Approval of the final version of the manuscript to be published
Authors’ Details
Abdullah Qamar1, Shabnam Hamid2, Fareeha Mushtaq3, Muhammad Saad Abdullah4, Faiza Umbreen5, Rabya Khalid6
- Associate Professor, Department of Anatomy, Army Medical College, Rawalpindi, Pakistan
- Professor, Department of Anatomy, Army Medical College Rawalpindi, Rawalpindi, Pakistan
- Associate Professor, Department of Anatomy, Rawal Institute of Health Sciences, Islamabad, Pakistan
- Associate Professor, Department of Anatomy, Combined Military Hospital Kharian Medical College, Kharian, Pakistan
- Assistant Professor, Department of Anatomy, Army Medical College, Rawalpindi, Pakistan
- Demonstrator, Department of Anatomy, Army Medical College, Rawalpindi, Pakistan
References
- Parsamanesh N, Moossavi M, Bahrami A, Fereidouni M, Barreto G, Sahebkar A. NLRP3 inflammasome as a treatment target in atherosclerosis: a focus on statin therapy. Int Immunopharmacol. 2019;73:146–55. https://doi.org/10.1016/j.intimp.2019.05.006
- Attardo S, Musumeci O, Velardo D, Toscano A. Statins neuromuscular adverse effects. Int J Mol Sci. 2022;23(15):8364. https://doi.org/10.3390/ijms23158364
- 63rd Annual Conference of the Indian Society of Gastroenterology, ISGCON 2022-January 5th - 8th, 2023 in Jaipur. Indian J Gastroenterol. 2023;42(Suppl 1):1–124. doi: 10.1007/s12664-022-01305-9
- McKinnell IW, Parise G, Rudnicki MA. Muscle stem cells and regenerative myogenesis. Curr Top Dev Biol. 2005;71:113–30. https://doi.org/10.1016/S0070-2153(05)71004-8
- Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35(8):1151–6. https://doi.org/10.1016/s1357-2725(03)00042-6
- Alfaqih MS, Tarawan VM, Sylviana N, Goenawan H, Lesmana R, Susianti S. Effects of vitamin D on satellite cells: a systematic review of in vivo studies. Nutrients. 2022;14(21):4558. https://doi.org/10.3390/nu14214558
- Vicente-García C, Hernández-Camacho JD, Carvajal JJ. Regulation of myogenic gene expression. Exp Cell Res. 2022;419(1):113299. https://doi.org/10.1016/j.yexcr.2022.113299
- Asfour HA, Allouh MZ, Said RS. Myogenic regulatory factors: the orchestrators of myogenesis after 30 years of discovery. Exp Biol Med (Maywood). 2018;243(2):118–28. https://doi.org/10.1177/1535370217749494
- Kaczmarek A, Kaczmarek M, Ciałowicz M, Clemente FM, Wolański P, Badicu G, et al. The role of satellite cells in skeletal muscle regeneration-the effect of exercise and age. Biology. 2021;10(10):1056. https://doi.org/10.3390/biology10101056
- Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280(17):4294–314. https://doi.org/10.1111/febs.12253
- Koopman R, Gehrig SM, Léger B, Trieu J, Walrand S, Murphy KT, et al. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic {beta}-adrenoceptor stimulation in mice. J Physiol. 2010;588(Pt 23):4811–23. https://doi.org/10.1113/jphysiol.2010.196725
- Laganá G, Barreca D, Calderaro A, Bellocco E. Lactate dehydrogenase inhibition: biochemical relevance and therapeutical potential. Curr Med Chem. 2019;26(18):3242–52. https://doi.org/10.2174/0929867324666170209103444
- Klein R, Nagy O, Tóthová C, Chovanová F. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet Med Int. 2020;2020:e5346483. https://doi.org/ 0.1155/2020/5346483
- Mahmoud Amany R, Kamel Esam O, Ahmed Marwa A, Ahmed Esraa A, Abd-Elhamid Tarek H. Alleviation of simvastatin-induced myopathy in rats by the standardized extract of Ginkgo Biloba (EGb761): insights into the mechanisms of action. Cells Tissues Organs. 2019;208(3-4):158–76. https://doi.org/10.1159/000507048
- Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. https://doi.org/ 10.4103/0976-0105.177703
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191(2):270–83. https://doi.org/10.1006/dbio.1997.8721
- Ji S, Ma P, Cao X, Wang J, Yu X, Luo X, et al. Myoblast-derived exosomes promote the repair and regeneration of injured skeletal muscle in mice. FEBS Open Bio. 2022;12(12):2213–26. https://doi.org/10.1002/2211-5463.13504
- Isesele PO, Mazurak VC. Regulation of skeletal muscle satellite cell differentiation by omega-3 polyunsaturated fatty acids: a critical review. Front Physiol. 2021;12:e682091. https://doi.org/10.3389/fphys.2021.682091
- Dorotea D, Ha H. Activation of β2 adrenergic receptor signaling modulates inflammation: a target limiting the progression of kidney diseases. Arch Pharm Res. 2021;44(1):49–62. https://doi.org/ 10.1007/s12272-020-01280-9
- Gómez-SanMiguel AB, Gomez-Moreira C, Nieto-Bona MP, Fernández-Galaz C, Villanúa M, Martín AI, et al. Formoterol decreases muscle wasting as well as inflammation in the rat model of rheumatoid arthritis. Am J Physiol Endocrinol Metab. 2016;310(11):e925–37. https://doi.org/10.1152/ajpendo.00503.2015
- Martín AI, Gómez-SanMiguel AB, Priego T, López-Calderón A. Formoterol treatment prevents the effects of endotoxin on muscle TNF/NF-kB, Akt/mTOR, and proteolytic pathways in a rat model. Role of IGF-I and miRNA 29b. Am J Physiol Endocrinol Metab. 2018;315(4):e705–14. https://doi.org/10.1152/ajpendo.00043.2018
- Tashkin DP. Formoterol for the treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:3105–22. https://doi.org/10.2147/COPD.S273497
- Yu D, Cai Z, Li D, Zhang Y, He M, Yang Y, et al. Myogenic differentiation of stem cells for skeletal muscle regeneration. Stem Cells Int. 2021;2021:e8884283. https://doi.org/10.1155/2021/8884283
- Salazar-Degracia A, Busquets S, Argilés JM, López-Soriano FJ, Barreiro E. Formoterol attenuates increased oxidative stress and myosin protein loss in respiratory and limb muscles of cancer cachectic rats. Peer J. 2017;5:e4109. https://doi.org/10.7717/peerj.4109
Keywords: Statin myotoxicity, β2-agonists, Formoterol, MyoD, LDH
Publication History
Received: July 08, 2023
Revised: October 31, 2023
Accepted: November 11, 2023
Published: December 25, 2023
Authors
Abdullah Qamar
Associate Professor, Anatomy Department, Army Medical College, Rawalpindi, Pakistan.
Shabnam Hamid
Professor, Anatomy Department, Army Medical College, Rawalpindi, Pakistan.
Fareeha Mushtaq
Associate Professor, Anatomy Department, Rawal Institute of Health Sciences, Islamabad, Pakistan.
Muhammad Saad Abdullah
Associate Professor, Anatomy Department, CMH Kharian Medical College, Kharian, Pakistan.
Faiza Umbreen
Assistant Professor, Department of Anatomy, Army Medical College, Rawalpindi, Pakistan.
Rabya Khalid
Demonstrator, Department of Anatomy, Army Medical College, Rawalpindi, Pakistan.
