Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(2):105-109
ORIGINAL ARTICLE
Effect of postoperative analgesia with the combination of dexmedetomidine and butorphanol after posterior spinal surgery
Qiaoling Wu1, You Shang1, Yanli Bai1, Yuan-yuan Wu1, Hao Wang1, Tu Shen1
Received: 19 February 2021 Revised date: 16 May 2021 Accepted: 03 May 2021
Correspondence to: Tu Shen
*Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University, Jinzhou City, China.
Email: st1212@tom.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Opioid medications used to be the key method for the pain management after spine surgery; most of the opioids may cause many adverse reactions. The purpose of this research was to observe the role of dexmedetomidine combined with butorphanol in the pain management of patients after posterior spinal surgery.
Methods:
This research was conducted at the First Hospital Affiliated to Jinzhou Medical University, China from May 2018 to January 2019. Sixty patients who underwent posterior spinal surgery were equally divided into two groups randomly: Group B who received butorphanol 0.125 mg/kg and Group DB who received dexmedetomidine (DEX) 0.1 μg/kg/hour plus butorphanol (B) 0.125 mg/kg. The patient-controlled analgesia was conducted to deliver a bolus dose of 0.5 ml. followed by an infusion of 2 ml/hour and a lockout time of 15 minutes nutes. Heart rate, mean arterial pressure, respiration rate, pulse oxygen saturation, visual analog scale score (VAS), and Ramsay sedation score were recorded as follows: 1 hour (T1), 2 hours (T2), 6 hours (T3), 12 hours (T4), and 24 hours (T5) post-surgery. The total number of buttons pressing of patient controlled intravenous analgesia (PCIA) and supplementary analgesic agents was observed and adverse drug reactions and total rate of patient satisfaction were evaluated statistically.
Results:
VAS scores at different intervals in DB group were significantly lower compared with the B group after surgery; while the score of Ramsay sedation was remarkably higher in DB group. The total number of buttons pressing of PCIA was less than that of the B group and the frequency of nausea was notably lower in DB group (p < 0.05). The total rate of satisfaction with analgesia in DB group was higher after surgery.
Conclusion:
DEX could enhance the analgesic effect of butorphanol after posterior spinal surgery with lesser adverse reactions.
Keywords:
Dexmedetomidine; Butorphanol; Posterior spinal surgery; Postoperative analgesia.
Introduction
Optimal postoperative pain control can lead to reduced complications, faster recovery, as well as improved patient’s quality of life. The opioid medications once used to be the key method for pain management after spine surgery [1] but most of the opioids may cause many adverse reactions including postoperative nausea and vomiting (PONV), pruritus, and urinary retention [2]. To resolve this issue, many new methods and new better anesthetics were developed [3,4]. Among the anesthetic, butorphanol is widely used due to its partial activities of agonist/antagonist on μ-opioid receptors. It has more powerful analgesia efficiency and lesser adverse reactions than morphine [5-8]. However, some serious adverse effects are also observed with this drug such as excessive sedation, respiratory depression, PONV and dizziness [9]. Therefore, it seems necessary to offer drug in combination to reduce these adverse reactions
Dexmedetomidine (DEX), a novel selective α-2 adrenergic receptor agonist which does not cause respiratory depression, is mainly applied in intensive care for sedation or analgesia, but it does not cause respiratory depression [10]. DEX as an adjunct with intravenous patient-controlled analgesia (IV-PCA) opioids (morphine, fentanyl, sufentanil, and butorphanol) has been confirmed to reduce opioids related side effects, provide better patients’ satisfaction undergoing abdominal total hysterectomy or partial laryngectomy or laparoscopic nephrectomy or total hysterectomy under laparoscopy [11-15]. However, up to now, studies focusing on combination of DEX and butorphanol are still inadequate. The primary purpose of this research was to evaluate the safety and effectiveness for combination use of DEX and butorphanol for postoperative analgesia in patients undergoing posterior spinal surgery.
Methods
Sixty patients (aged 25-58 years) with American Society of Anesthesiologist physical status I or II and body mass index <28 kg/m2 undergoing posterior spinal surgery were included. The following patients were excluded: 1) patients with hypertension or ischemic heart disease; 2) patients who had taken β-adrenoreceptor blockers or analgesics 1 week before the study; and 3) patients with neuropsychiatric diseases, conduction abnormality, or allergy to butorphanol or DEX. All patients signed the informed consent form and the research was approved by Ethics Committee of the First Hospital Affiliated to Jinzhou Medical University, China vide Project No. 20180305.
Patients were continuously monitored through electrocardiography, blood pressure (BP), pulse oxygen saturation (SpO2), end-tidal carbon dioxide before the operation. Before anesthetic induction, midazolam 0.04 mg/kg was intravenously injected into all patients and penehyclidine hydrochloride 0.5 mg before anesthetic induction was given. For anesthetic induction, etomidate (0.3 mg/kg), sufentanil (0.4 g/kg) and cisatracurium (0.15 mg/kg) were intravenously injected. Sevoflurane (1.0-2.5MAC) was used to maintain anesthesia and an intravenous infusion of DEX 0.4 μg/kg/hour combined with remifentanil (0.1-0.2 μg/kg/minute). Cisatracurium (0.05 mg/kg) was managed during operation or 1 hour before the end of operation. Hemodynamic stability was maintained intraoperatively.
All patients received an intravenous injection of Butorphanol 1mg and toxanisqiong 2 mg 15 minutes before completion of the surgery. An electronic infusion pump for intravenous PCA was used for all patients after surgery. The patients were administered butorphanol 0.125 mg/kg in the group B. The patients were administrated DEX 0.1 ug/kg/hour plus butorphanol 0.125 mg/kg in the group DB. 0.9% normal saline was added to prepare 100 ml PCA and infused with a 0.5 ml bolus on-demand.
The determination of vital signs was made to ensure the safety of the patients. The physiological indices included heart rate (HR), mean arterial pressure (MAP), respiration rate (RR), and SpO2. 1 hour (T1), 2 hours (T2), 6 hours (T3), 12 hours (T4), and 24 hours (T5) post-surgery of HR, MAP, RR, and SpO2 were recorded.
The VAS score was analyzed by standard criteria: 0: painless, 1-4: mild pain, 5-6: moderate pain, and 7-10: severe pain. VAS scores at rest and movement were recorded at T1-T5.
Ramsay sedation score was calculated as 1: fidgety; 2: quite cooperative; 3: responds quickly to the instruction, but the pronunciation is ambiguous; 4: asleep, easily wake up by light tactile stimulation or the simple verbal commands; 5: drowsy, slow response to instructions; and 6: deep sleep. The score between 2 ~ 4 represent satisfactory sedation; score of 5-6 represent excessive sedation. Ramsay sedation score were recorded at T1-T5.
The total number of buttons pressing of PCIA within 24 hours was observed, the number of supplementary analgesic agents was recorded, other adverse drug reactions (nausea, vomiting, bradycardia, respiratory depression) were noted, and total rate of patient satisfaction (very satisfied, satisfied, somewhat satisfied, not satisfied) were evaluated statistically.
Statistical analysis
Statistical analysis was conducted using Statistical Package for the Social Sciences 22.0 (SPSS Inc. Chicago, IL). Data were shown as the mean ± SD (standard deviation). Comparison between the 2 groups was analyzed using independent student’s t-tests and comparison among groups was conducted using 2-way analysis of variance. p < 0.05 was considered as statistically significant.
Results
As shown in Table 1, there was no difference in patient gender, age, or weight among the study groups. No statistically significant difference was found between the two groups at T1-T5 with HR, MAP, RR, SpO2 (Table 2).
Patients showed an obviously lower VAS score in the DB group both at rest and movement than the group B (p < 0.05) (Figure 1). Postoperative Ramsay sedation score of DB group was higher compared with the group B at T1, T2, T3 and T4, but there was no significant difference (Figure 2). Patients in the B group (35 ± 8) demonstrated a significantly higher pump-press number than the DB (8 ± 3) group (p < 0.05).
Table 1. Patient characteristics in the two groups (n = 30).
| Variables | Group B | Group DB |
|---|---|---|
| Age (years) | 45.17 ± 9.49 | 44.67 ± 9.38 |
| Weight (kg) | 63.70 ± 7.91 | 64.27 ± 7.79 |
| BMI (kg/ m2) | 22.4 ± 2.8 | 23.1 ± 2.3 |
Values are expressed as the mean ± standard deviation.
The group DB showed a lower frequency of nausea than the group B (p < 0.05), and no obvious difference occurred in the frequency of vomiting and bradycardia between the two groups, and the frequency of respiratory depression was zero (Table 3).
Furthermore, results indicated that total rate of patients’ satisfaction was obviously greater in the DB group than that of group B (p < 0.05) (Table 4).
Discussion
In order to alleviate postoperative pain, postoperative analgesia is a vital component [16,17]. PCIA is an effective method in reducing the severe pain after the spinal surgery. However, the application of morphine drugs in PCIA has many side effects, and thus needs to be improved. DEX is recently reported to be used in the treatment of many diseases and postoperative treatments [18]. Combining DEX with opioids in PCIA after the surgery has reported to reduce opioids consumption and improved patients’ satisfaction compared with opioids in PCIA alone [13,14]. In this study, combination treatment of DEX 0.1 μg/kg/hour and butorphanol 0.125 mg/kg enhanced the analgesic effect of single use of butorphanol, and decreased the rate of the butorphanol-induced PONV and some severe adverse reactions.
Butorphanol is a commonly used opioid analgesic for postoperative PCIA, and also is a lipid-soluble narcotic agent that has strong κ-receptor agonist and weak μ-receptor agonist/antagonist activity [8]. In this study, it is indicated that combination of DEX and butorphanol demonstrate an obviously lower VAS score at rest and movement and a significantly lower pump-press number compared with butorphanol alone. DEX demonstrated to strengthen the analgesic role of butorphanol and lessen the required dosage of butorphanol. The results of this study also confirm that DEX can enhance the analgesic effect of opioids and reduce their consumption [15,18,19]. The underlying mechanism may be a synergistic analgesic effect of DEX and butorphanol that can produce effective analgesia when combined [20].
However, high dosage of butorphanol can produce narcolepsy, dizziness, inhalation inhibition, nausea, vomiting and other adverse reactions. In the present research, combination of DEX and butorphanol demonstrated a lower frequency of nausea compared with butorphanol alone. It may be due to the antiemetic effect of DEX due to its inhibitory effect on adrenergic receptors and sedation effect [21]. It is also likely attributable that the combination treatment reduced the dosage of butorphanol [8]. In other words, the dose of butorphanol was reduced with subsequent reduction in the frequency of vomiting.
Table 2. Comparison of vital signs between the two groups (n = 30).
| Group | T1 | T2 | T3 | T4 | T5 | |
|---|---|---|---|---|---|---|
| HR (bpm) | B | 71 ± 13 | 74 ± 15 | 76 ± 10 | 74 ± 6 | 73 ± 6 |
| DB | 72 ± 15 | 73 ± 17 | 72 ± 14 | 70 ± 10 | 74 ± 10 | |
| MAP (mm Hg) | B | 74 ± 10 | 69 ± 8 | 69 ± 6 | 71 ± 5 | 69 ± 6 |
| DB | 73 ± 6 | 68 ± 5 | 66 ± 5 | 68 ± 6 | 69 ± 6 | |
| RR (bpm) | B | 18 ± 2 | 17 ± 3 | 18 ± 1 | 18 ± 2 | 18 ± 1 |
| DB | 18 ± 3 | 17 ± 2 | 17 ± 2 | 17 ± 1 | 17 ± 2 | |
| SpO2 (%) | B | 98 ± 1 | 97 ± 2 | 98 ± 3 | 97 ± 1 | 97 ± 3 |
| DB | 97 ± 2 | 97 ± 2 | 97 ± 3 | 98 ± 2 | 97 ± 2 |
Values are expressed as the mean ± standard deviation.
HR = heart rate, MAP = mean arterial pressure, RR = respiration rate, SpO2 = oxygen saturation.
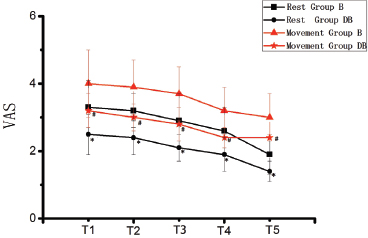
Figure 1. VAS pain score (at rest and movement) at different time points in the two groups. *p < 0.05 compared with group B at rest; #p < 0.05 compared with group B at movement.
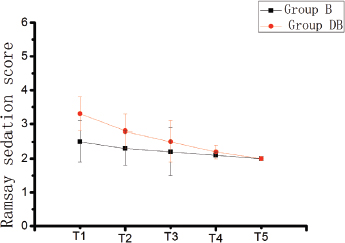
Figure 2. Ramsay sedation score at different time points in the two groups. Compared with group B, the group DB showed lower frequency of nausea (p < 0.05).
Table 3. Comparison of adverse reactions between the two groups [cases (%), n = 30].
| Group | Nausea | Vomiting | Bradycardia | Respiratory depression |
|---|---|---|---|---|
| B | 8 (26.7) | 1 (3.3) | 1 (3.3) | 0 |
| DB | 2 (6.7)* | 1 (3.3) | 1 (3.3) | 0 |
Data indicated the number and percentage of patients, n (%).
*p < 0.05 compared with group B
Table 4. Comparison of total rate of patient satisfaction between the two groups [cases (%), n = 30].
| Group | Very satisfied | Satisfied | Somewhat satisfied | Not satisfied | Satisfaction |
|---|---|---|---|---|---|
| B | 8 (26.7) | 5 (16.7) | 10 (33.3) | 7 (23.3) | 23 (76.7) |
| DB | 12 (40) | 8 (26.7) | 8 (26.7) | 2 (6.7) | 28 (93.3*) |
Data indicated the number and percentage of patients, n (%).
*p < 0.05 compared with group B.
Moderate postoperative sedation can relieve the tension and anxiety of the patients and contribute to postoperative rehabilitation. Butorphanol produces a sedative effect by acting on κ-receptor, and DEX also has a sedative effect. However, in this study, the sedative process of the patients was appropriate, and there was no obvious difference in the sedative score between the two groups. It may be because the dose of DEX in this study was lower than the conventional sedative dose 0.2-0.7 (μg/kg/hour), [22] and adding DEX to butorphanol PCA reduced butorphanol dosage and corresponding sedative effect.
Conclusion
DEX can enhance the postoperative analgesic effect of butorphanol in patients undergoing spinal surgery, reduce the consumption of butorphanol with subsequent lower frequency of reported adverse reactions while improving patients’ satisfaction at the same time. An important part of this process is the reduction of postoperative pain, the ability to get out of the bed early and the promotion of early recovery. However, the selection of the dose of DEX remains to be further studied.
Limitations of the study
The current research has certain limitations. First, only one dose of DEX was used, whether higher or a lower dose could produce the similar or better effect, remains to be further studied. Second, only one type of orthopedic surgery was performed; hence, further studies with inclusion of other major types of surgeries is suggested in order to widen the scope of these drugs for postoperative analgesia.
Acknowledgement
The authors would like to thank the surgeons, anesthesiologists, and nurses of the First Hospital Affiliated to Jinzhou Medical University China who participated in this study.
Conflict of interest
None to declare.
Grant Support & financial disclosure
None to disclose
Ethical approval
The protocol of this research was agreed to the Ethics Committee of the First Hospital Affiliated to Jinzhou Medical University. All participants have agreed and signed the informed consent form. (Approval No. 412/19)
Authors contribution
QW, YS, YW, HW, and TS: Conception and design of study, acquisition and analysis of data, drafting and critical review of the manuscript.
YB: Critical revision of manuscript for important intellectual content.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Author details
Qiaoling Wu1, You Shang1, Yanli Bai1, Yuan-yuan Wu1, Hao Wang1, Tu Shen1
- Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University, China
References
- Rainov NG, Gutjahr T, Burkert W. Intra-operative epidural morphine, fentanyl, and droperidol for control of pain after spinal surgery. A prospective, randomized, placebo-controlled, and double-blind trial. Acta Neurochir (Wien). 1996;138(1):33–9. https://doi.org/10.1007/bf01411721
- Walder B, Schafer M, Henzi I, Tramèr MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand. 2001;45(7):795–4. https://doi.org/10.1034/j.1399-6576.2001.045007795.x
- Kurd MF, Kreitz T, Schroeder G, Vaccaro AR. The role of multimodal analgesia in spine surgery. J Am Acad Orthop Surg. 2017;25(4):260–8. https://doi.org/10.5435/JAAOS-D-16-00049
- Bedin A, Caldart Bedin RA, Vieira JE, Ashmawi HA. Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. 2017;33(10):865–9. https://doi.org/10.1097/AJP.0000000000000471
- Sellon DC, Monroe VL, Roberts MC, Papich MG. Pharmacokinetics and adverse effects of butorphanol administered by single intravenous injection or continuous intravenous infusion in horses. Am J Vet Res. 2001;62(2):183–9. https://doi.org/10.2460/ajvr.2001.62.183
- Du BX, Song ZM, Wang K, Zhang H, Xu FY, Zou Z, et al. Butorphanol prevents morphine-induced pruritus without increasing pain and other side effects: a systematic review of randomized controlled trials. Can J Anaesth. 2013;60(9):907–17. https://doi.org/10.1007/s12630-013-9989-4
- Wang F, Shen X, Liu Y, Xu S, Guo X. Continuous infusion of butorphanol combined with intravenous morphine patient-controlled analgesia after total abdominal hysterectomy: a randomized, double-blind controlled trial. Eur J Anaesthesiol. 2009;26(1):28–34. https://doi.org/10.1097/EJA.0b013e32831a6aa2
- Zhang XK, Chen QH, Wang WX, Hu Q. Evaluation of dexmedetomidine in combination with sufentanil or butorphanol for postoperative analgesia in patients undergoing laparoscopic resection of gastrointestinal tumors: a quasi-experimental trial. Medicine (Baltimore). 2016;95(50):e5604. https://doi.org/10.1097/MD.0000000000005604
- Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol. 2006;54(3):527–31. https://doi.org/10.1016/j.jaad.2005.12.010
- Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116(6):1312–22. https://doi.org/10.1097/ALN.0b013e31825681cb
- Lin TF, Yeh YC, Lin FS, Wang YP, Lin CJ, Sun WZ, et al. Effect of combining dexmedetomidine and morphine for intravenous patient-controlled analgesia. Br J Anaesth. 2009;102(1):117–22. https://doi.org/10.1093/bja/aen320
- Kim SY, Chang CH, Lee JS, Kim YJ, Kim MD, Han DW. Comparison of the efficacy of dexmedetomidine plus fentanyl patient-controlled analgesia with fentanyl patient-controlled analgesia for pain control in uterine artery embolization for symptomatic fibroid tumors or adenomyosis: a prospective, randomized study. J Vasc Interv Radiol. 2013;24(6):779–86. https://doi.org/10.1016/j.jvir.2013.02.034
- Qin M, Chen K, Liu T, Shen X. Dexmedetomidine in combination with sufentanil for postoperative analgesia after partial laryngectomy. BMC Anesthesiol. 2017;17(1):66. https://doi.org/10.1186/s12871-017-0363-x
- Song F, Ye C, Qi F, Zhang P, Wang X, Lü Y, et al. Effect of perioperative infusion of dexmedetomidine combined with sufentanil on quality of postoperative analgesia in patients undergoing laparoscopic nephrectomy: a CONSORT-prospective, randomized, controlled trial. BMC Anesthesiol. 2018;18(1):145. https://doi.org/10.1186/s12871-018-0608-3
- Du J, Li JW, Jin J, Shi CX, Ma JH. Intraoperative and postoperative infusion of dexmedetomidine combined with intravenous butorphanol patient-controlled analgesia following total hysterectomy under laparoscopy. Exp Ther Med. 2018;16(5):4063–9. https://doi.org/10.3892/etm.2018.6736
- Schenk MR, Putzier M, Kügler B, Tohtz S, Voigt K, Schink T, et al. Postoperative analgesia after major spine surgery: patient-controlled epidural analgesia versus patient-controlled intravenous analgesia. Anesth Analg. 2006,103(5):1311–7. https://doi.org/10.1213/01.ane/0000247966.49492.72.
- Tian P, Fu X, Li ZJ, Ma XL.Comparison of patient-controlled epidural analgesia and patient-controlled intravenous analgesia after spinal fusion surgery: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2015;16(15):388. https://doi.org/10.1186/s12891-015-0849-y
- Dong CS, Lu Y, Zhang J, Sun P, Yu JM, Wu C, et al. The optimal dose of dexmedetomidine added to an sufentanil-based analgesic regimen for postoperative pain control in spine surgery: a probit analysis study. Medicine (Baltimore). 2016;95(39):e4776. https://doi.org/10.1097/MD.0000000000004776
- Kweon DE, Koo Y, Lee S, Chung K, Ahn S, Park C. Postoperative infusion of a low dose of dexmedetomidine reduces intravenous consumption of sufentanil in patient-controlled analgesia. Korean J Anesthesiol. 2018;71(3):226–31. https://doi.org/10.4097/kja.d.18.27056
- Unlugenc H, Gunduz M, Guler T, Yagmur O, Isik G. The effect of pre-anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient-controlled morphine. Eur J Anaesthesiol. 2005;22(5):386–91. https://doi.org/10.1017/s0265021505000669
- Song Y, Shim JK, Song JW, Kim EK, Kwak YL. Dexmedetomidine added to an opioid-based analgesic regimen for the prevention of postoperative nausea and vomiting in highly susceptible patients: a randomised controlled trial. Eur J Anaesthesiol. 2016;33(2):75–83. https://doi.org/10.1097/EJA.0000000000000327
- Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362–8. https://doi.org/10.1097/ALN.0000000000000951
Keywords: Dexmedetomidine; Butorphanol; Posterior spinal surgery; Postoperative analgesia.
Publication History
Received: February 19, 2021
Revised: May 16, 2021
Accepted: May 30, 2021
Published: June 30, 2021
Authors
Qiaoling Wu
Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University - China.
You Shang
Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University - China.
Yanli Bai
Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University - China.
Yuan-yuan Wu
Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University - China.
Hao Wang
Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University - China.
Tu Shen
Department of Anesthesiology, the First Hospital Affiliated to Jinzhou Medical University - China.
