Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(4):259-264
ORIGINAL ARTICLE
Serum PIVKA-II: reference interval of healthy population and establishment of its cutoff value for hepatocellular carcinoma diagnosis in Pakistan
Faryal Husnain1, Muhammad Dilawar Khan2, Omar Rasheed Chughtai3, Akhtar Sohail Chughtai4, Shakeel Ashraf5, Ahmed Yar5
Received: 08 July 2021 Revised date: 11 November 2021 Accepted: 12 December 2021
Correspondence to: Faryal Husnain
*Department of Chemical Pathology and Immunology, Chughtai Institute of Pathology, Lahore, Pakistan.
Email: drfaryalhusnain.5712@cll.edu.pk
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Protein induced by vitamin K absence II (PIVKA-II) is an upcoming and promising new biological marker cited as having a definitive role in the early detection and diagnosis of hepatocellular carcinoma (HCC). Preliminary research shows PIVKA-II reference intervals (RIs) in serum to have substantial racial disparities globally. Hence, this study aimed to determine the RIs and cut-off value of the serum PIVKA-II for the first time in healthy and patients with HCC in Pakistan.
Methods:
This cross-sectional study comprised 240 participants (120 diagnosed cases of HCC and 120 healthy individuals) registered at the Department of Chemical Pathology and Immunology, Chughtai Institute of Pathology, Lahore, Pakistan. The PIVKA-II serum level was analyzed using the Chemistry Analyzer through chemiluminescent micro-particle immunoassay. The RI was subsequently established using the percentile method.
Results:
In healthy Pakistani adults, the 95% RI for PIVKA-II was 15.55-43.03 mAU/ml, and the cut-off was 148.81 mAU/ml in HCC cases. The male participants, exhibited higher PIVKA-II levels than the females (p < 0.002). There were no significant differences in the serum levels of PIVKA-II with respect to age however variations were observed with respect to gender.
Conclusion:
Determination of the RI for serum PIVKA-II in healthy adult Pakistani individuals and a cut-off for HCC diagnosis emphasizes the growing need to establish and verify RIs of analytes at a larger scale in our population. Ideally all clinical laboratories should establish their own RIs.
Keywords:
Hepatocellular carcinoma, alpha feto protein, PIVKA-II, reference interval, cut-off value, healthy controls.
Introduction
Reference intervals (RIs) allow physicians to make prompt and accurate diagnosis and also interpret laboratory findings during the course of disease with a certain level of confidence.1
These are crucial for patients’ physiological evaluation, for making a diagnosis or therapeutic management decisions and for monitoring their prognosis. 1-3
There is a growing need to establish and verify RIs of analytes in different populations, owing to different racial and ethnic groups and diverse analytical methodologies used in different laboratories for a single biomarker. Ideally, all clinical laboratories should establish their own RIs. 4,5
It is recommended to establish RIs using standard procedure as outlined by Clinical Laboratory and Standards Institute recommendations. 6,7
Globally hepatocellular carcinoma (HCC) is the commonest primary malignant neoplasm of the liver and is responsible for significant cancer-mortality. It’s estimated to be the fourth most common etiology of all cancer associated mortality, the first three being lung, colorectal, and stomach cancer, respectively. 8 Pakistan has an alarmingly high prevalence rate (3.7%-16%), there is a significant male preponderance similar to the global pattern with the female-to-male ratio at 10:36. 9 In Pakistan, cirrhosis is the leading cause of HCC; the etiology of the majority (87%) of cases of hepatic cirrhosis can be attributed to viral hepatitis; out of which Hepatitis C contributes 68% and Hepatitis B contributes 22% of cases.10 Due to a paucity of reliable and advanced diagnostic radiology facilities in most of the rural areas in Pakistan with high burden of disease, majority of the cases are detected at a terminal phase of the disease. Unfortunately, less than 1% of the patients in Pakistan present to hospitals when the tumors are surgically resectable. 11 Screening for HCC usually comprises of two modalities; radiological examination (such as ultrasound, computerized tomography, and magnetic resonance imaging), and biochemical markers such as alpha-fetoprotein (AFP), both of which are recommended at 6-month intervals in patients with cirrhosis. 12 Protein induced by vitamin K absence-II (PIVKA II) is a promising new biomarker for early detection of HCC and could have a greater role in screening for HCC especially in Pakistan.
PIVKA-II also known by different names like Dicumarol-induced (vitamin K antagonist) prothrombin, or atypical prothrombin, Hepatoma-associated abnormal (des-γ-carboxy) prothrombin, des-γ-carboxyprothrombin, abnormal prothrombin, isoprothrombin, and dicumarolized prothrombin, is an abnormal variant of the clotting factor II or prothrombin. It is structurally very similar to prothrombin but has only 4% of the activity of normal purified prothrombin. In PIVKA-II, some residues (the 10 Glu residues are normally positioned at 6, 7, 14, 16, 19, 20, 25, 26, 29, and 32 in the N-terminal domain also called as gamma-Linolenic acid (GLA) domain) are not gamma carboxylated and hence it does not have the calcium binding properties necessary for its action. It was initially discovered in vitamin K deficient patients or patients taking a vitamin K antagonist like dicumarol. HCC cells produce large amounts of PIVKA-II instead of normal prothrombin and hence it is a valuable screening biomarker for HCC. The association between serum PIVKA II elevation and HCC was first reported by Liebman et al. 13
PIVKA-II’s diagnostic parameters like specificity and sensitivity have been proven to be better than AFP which was previously considered as the biomarker of choice for HCC. It has increasingly gained importance in the screening and diagnosis of HCC. 14-17
Currently, no data is available for the RIs of PIVKA-II levels in serum in the Pakistani populace, both in health and disease. According to the literature, 95% reference range shows a significant ethnic difference between different races e.g., the Japanese 11.12-32.01 mAU/ml (n = 193) and 17.36-50.90 mAU/ml Italians (European Union) (n = 435). 18,19 Significant disparities, hence, between different racial backgrounds underscores the need to determine and verify RIs for different populations. This study was, therefore, designed to determine the serum PIVKA-II RIs in healthy subjects and cut-off in cases with HCC in population of Lahore, Pakistan.
Methods
This cross-sectional study was performed at the Department of Chemical Pathology and Immunology, Chughtai Institute of Pathology, Lahore, Pakistan. It was carried out from January 14, 2020 to February 14, 2020 after getting an approval from the Institutional Ethical Review Board vide Letter No. CIP/IRB/1044. Inclusion criteria for patients with HCC was the patients diagnosed on biopsy; including both genders, adult age group, any grade, histological type or TNM stage of HCC. Patients with bleeding disorders and other debilitating chronic infectious or immune co-morbid conditions were excluded. Inclusion criteria for healthy control was subjects with no evidence of disease or family history of HCC at the time of presentation. Blood samples were collected from 120 diagnosed cases of HCC and 120 healthy individuals.
About 3 ml of venous blood sample was drawn from each subject (healthy and HCC cases) to obtain approximately 1.5 ml of serum. The sample was centrifuged at 4,000 revolutions per minute for 20 minutes. Serum samples that were purely hemorrhagic, hemolyzed, lipemic or icteric or with only fibrin or with any debris contamination were excluded and repeated till satisfactory sample was achieved.
These tests were performed on Chemistry Analyzer Alinity-I (Abbott) and the reagents used were PIVKA-II reagent kit (1R1732). This assay is a chemiluminescent micro-particle immunoassay technique.
Statistical analysis
Data were processed by Number Cruncher Statistical Systems (NCSS) software and the RIs and cut-off values for diagnosis of HCC were established. Mean, median, standard deviation (SD), and interquartile ranges (IQR) were determined for healthy subjects and HCC cases. Skewness and Shapiro-Wilk normality were also applied to both healthy and diseased subjects (n = 240) using the NCCS software. RIs in healthy individuals (n = 120) were established by the two-sided percentile method. The formula for this was given by Horn et al. 18 In our study, the upper and lower limits of the RIs are defined as 100 (1 -α/2) and 100 (α/2) percentiles of the sorted data values respectively. The methods for percentile estimation are listed as follows: Method A: p (n + 1) Method A, which calculates an index p (n + 1). The data in HCC cases were not normally distributed so the RIs in diseased (HCC) cases (n = 120) were calculated by an alternative method. It is a modified percentile method which involves a Box-Cox transformation to make the data distribution more amenable to the formula. The formula for this method is as given: the lower and upper limits of the RIs are defined as RL = x̄ + ta/2, n-1 ^s √1+1/n Ru = x̄+ t1 -a/2, n-1^s √1+1/n, where x̄ is the sample mean and s is the sample SD. The data were analyzed using NCSS version 2021 computer software.
Results
Mean age of the 240 subjects was 39.5 ± 13.4 years; 165 males and 74 females from Lahore were finally included in this analysis. The RI of PIVKA-II was 15.55-43.03 mAU/ml (95%) in healthy subjects and the cut-off for the diagnosis was 148.810 mAU/ ml in HCC cases. Both of these were calculated by the percentile method. The RIs were determined by using the two-sided (2.5th to the 97.5th) percentile range of the cases in healthy individuals and the one-sided (lower 5th percentile) cut-off for the diagnosis of HCC cases (Tables 1 and 2 respectively). The distribution of values of serum PIVKA II in healthy adults and HCC cases is shown in Figures 1 and 2.
Discussion
The establishment of a RI for a biological marker is crucial for its clinical use in the diagnosis and prognosis of a disease with critical value as a biomarker during medical decision making. 4-6 Unavailability of gender, age, ethnic, regional, and methodology specific RIs affect medical decision making and interventional strategies in healthcare settings. 19,4 PIVKA-II is a novel biomarker for the screening and diagnosis of HCC, but limited information and paucity of formal extended studies for determination of its RI in healthy populations limits its utility.
Table 1. Descriptive statistics of serum PIVKA II in healthy individuals (n = 120).
| Descriptive statistics of PIVKA II in healthy subjects (n = 120) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Count | Mean | Median | SD | IQR | Minimum | Maximum |
| PIVKAII | 120 | 28.759 | 28.665 | 6.9486 | 9.860 | 14.860 | 43.480 |
| Normality report of PIVKA II | |||||||
| Variable | Mean | SD | coefficient of variation | Skewness (normal = 0) | Kurtosis (normal = 3) | Anderson darling normality p-value | Shapiro Wilk normality p-value |
| PIVKAII | 28.759 | 6.9486 | 0.2416 | 0.0852 | 2.4716 | 0.7057 | 0.2119 |
| Quantile report of PIVKA II | |||||||
| Variable | 5th percentile | 10th percentile | 25th percentile | 50th percentile | 75th percentile | 90th percentile | 95th percentile |
| PIVKA II | 17.473 | 18.750 | 23.840 | 28.665 | 33.700 | 38.394 | 41.814 |
| Two-sided 95% percentile RI of PIVKA II | |||||||
| 2.5% lower reference limit (95% CI) | 97.5% upper reference limit (95% CI) | ||||||
| Variable | Count | Value | Variable | Count | Value | ||
| PIVKA II | 120 | 15.554 | PIVKA II | 120 | 43.031 | ||
Table 2. Statistics for cut-off of diagnostic cut-off for HCC (n = 120).
| Descriptive statistics of PIVKA II in HCC cases (n = 120) | ||||||
|---|---|---|---|---|---|---|
| Count | Mean | Median | SD | IQR | Minimum | Maximum |
| 120 | 8,390.447 | 4,006.20 | 8,951.8480 | 13,491.600 | 99.230 | 29,807.800 |
| Normality report of PIVKA II | ||||||
| Mean | SD | coefficient of variation | Skewness (normal = 0) | Kurtosis (normal = 3) | Anderson darling normality p-value | Shapiro Wilk normality p-value |
| 8,390.447 | 8,951.8480 | 1.0669 | 1.0445 | 2.8894 | 0.0000 | 0.0000 |
| Quantile report of PIVKA II | ||||||
| 5th percentile | 10th percentile | 25th percentile | 50th percentile | 75th percentile | 90th percentile | 95th percentile |
| 148.810 | 211.950 | 942.100 | 4,006.200 | 14,433.700 | 25,808.600 | 29,073.700 |
| Lower 5% percentile reference limit of PIVKA II | ||||||
| 5% lower reference limit (95% CI) | ||||||
| Variable | Count | Value | Upper | Lower | ||
| PIVKA II | 120 | 148.810 | 99.230 | 211.950 | ||
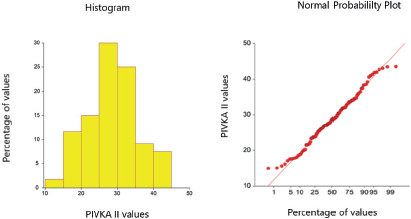
Figure 1. Scattering of serum PIVKA II values in healthy individuals (n = 120).
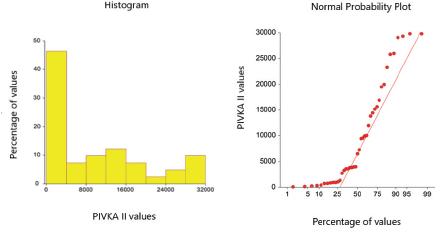
Figure 2. Scattering of serum PIVKA II values in HCC cases (n = 120).
Other studies have calculated the RI in different populations: Europeans (17.36-50.90 mAU/ml), Chinese (13.62-40.38 mAU/ml) and Japanese (11.12-32.01 mAU/ml). This shows that there are ethnic differences and that every country should establish their own RIs considering different ethnic groups within each population.
Different studies have determined cut-off for diagnosis of HCC. A recent study from India calculated it as 250 mAU/l 20 while a Korean study calculated it as 271.8 mAU/l 21 using receiver operating curve (ROC) curve analysis. Another study from Italy calculated it using mean +3 SD of PIVKA II levels in healthy population as 70 mAU/l. 22
Our study will add to the library of RIs in our local population. This data will facilitate further studies in Pakistani population.
Conclusion
Population-specific RIs are crucial for accurate detection of disease and decisions regarding patients’ treatment. Additional extended, large-scale, multicenter clinical studies for the determination of RIs of all analytes in population from different provinces of Pakistan are recommended to add-on to our findings.
Limitations of the study
There are some limitations in our study. First, all of our samples were collected from Lahore therefore it is not a good representation of all major ethnic populations of Pakistan, as our sample group included mostly Punjabis. Further experiments are warranted to determine the suitable RI in other racial sub-populations like Pakhtoon, Baloch, Sindhi, Kashmiri, Gilgiti and Urdu-speaking etc.
Additionally, as this is a cross-sectional analysis, it is not possible to determine whether there is any chronological change in PIVKA-II levels in subjects over certain time duration.
Furthermore, we chose diagnosed cases of HCC based on CT scans and biopsy findings, so we couldn’t use ROC analysis for determining cut-off for diagnosis; instead, we calculated our cut-off using the percentile method.
Acknowledgement
The authors would like to acknowledge all the staff and management of Chughtai Institute of Pathology, Lahore, Pakistan for their logistic and technical support.
List of Abbreviations
| AFP | Alpha fetoprotein |
| HCC | Hepatocellular carcinoma |
| mAU/ml | Milli absorption unit per milli liter |
| NCSS | Number Cruncher Statistical Systems |
| PIVKA II | Protein induced by vitamin K absence II |
| RI | Reference interval |
| ROC | Receiver operation curve |
| SD | Standard deviation |
Conflict of interest
None to declare.
Grant support and financial disclosure
This study was funded by the Chughtai Institute of Pathology Lahore, Pakistan.
Ethical approval
Ethical approval was granted by the Ethics Committee/Institutional Review Board of Chughtai Institute of Pathology Lahore, Pakistan via reference letter number 1044/20 dated: 30-09-2020.
Authors’ contribution
FH: Study design, drafting of manuscript, and acquisition of data.
MDK, ORC, and ARC: Concept of the study, data interpretation and critical intellectual input.
SA and AY: Data acquisition, analysis and interpretation, and final drafting of the manuscript.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ details
Faryal Husnain1, M Dilawar Khan2, Omar Rasheed Chughtai3, Akhtar Sohail Chughtai4, Shakeel Ashraf5, Ahmed Yar5
- Resident, Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan
- Professor, Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan
- Assistant Professor, Department of Histopathology, Chughtai Institute of Pathology, Lahore, Pakistan
- Chief Executive, Chughtai Institute of Pathology, Lahore, Pakistan
- Technologist, Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan
References
- Patel KK, Qavi AJ, Hock KG, Gronowski AM. Establishing reference intervals for HCG in postmenopausal women. Clin Biochem. 2017;50(4-5):234–7. https://doi.org/10.1016/j.clinbiochem.2016.11.017
- Jones GR, Koetsier S. Uptake of recommended common reference intervals for chemical pathology in Australia. Ann Clin Biochem. 2017;54(3):395–7. https://doi.org/10.1177/0004563216679853
- Sturgeon CM, Sprague S, Almond A, Cavalier E, Fraser WD, Algeciras-Schimnich A, et al. Perspective and priorities for improvement of parathyroid hormone (PTH) measurement-A view from the IFCC Working Group for PTH. Clin Chim Acta. 2017;467(5):42–7. https://doi.org/10.1016/j.cca.2016.10.016
- Ichihara K. Statistical considerations for harmonization of the global multicenter study on reference values. Clin Chim Acta. 2014;432(3):108–18. https://doi.org/10.1016/j.cca.2014.01.025
- Ichihara K, Ozarda Y, Barth JH, Klee G, Shimizu Y, Xia L, et al. A global multicenter study on reference values: 2. Exploration of sources of variation across the countries. Clin Chim Acta. 2017;467(6):83–97. https://doi.org/10.1016/j.cca.2016.09.015
- Horowitz GL, Altaie S, Boyd JC. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. CLSI; 2010. Available from: https://www.doi.org/researchgate.net/publication/281239796_Defining_ Establishing_and_Verifying_Reference_Intervals_in_the_Clinical_Laboratory_Approved_Guidelines_CLSI_document_C28-A3_Vol_28_No_3
- Ozarda Y, Ichihara K, Barth JH, Klee G. Protocol and standard operating procedures for common use in a worldwide multicenter study on reference values. CCLM. 2013;51(5):1027–40. https://doi.org/10.1515/cclm-2013-0249
- Stewart BW, Wild CP. World cancer report 2014. Lyon, France: IARC; 2014. 630 p. Available from: https://www.doi.org/publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014
- Butt AS, Abbas Z, Jafri W. Hepatocellular carcinoma in Pakistan: where do we stand? Hepat Mon. 2012;12(10 HCC):e6023. https://doi.org/10.5812/hepatmon.6023
- Munaf A, Memon MS, Kumar P, Ahmed S, Kumar MB. Comparison of viral hepatitis-associated hepatocellular carcinoma due to HBV and HCV-cohort from liver clinics in Pakistan. APJCP. 2014;15(18):7563–7. https://doi.org/10.7314/APJCP.2014.15.18.7563
- Butt AS, Hamid S, Wadalawala AA, Ghufran M, Javed AA, Farooq O, Ahmed B, et al. Hepatocellular carcinoma in native South Asian Pakistani population; trends, clinico-pathological characteristics & differences in viral marker negative & viral-hepatocellular carcinoma. BMC Res. 2013;6(1):1–3. https://doi.org/10.1186/1756-0500-6-137
- Diagnosis of hepatocellular carcinoma. HPB (Oxford). 2005;7(1):26–34. https://doi.org/10.1080/13651820410024049
- Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, et al. Des-γ-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. NEJM. 1984;31;310(22):1427–31. https://doi.org/10.1056/NEJM198405313102204
- Cui SX, Yu XF, Qu XJ. Roles and signaling pathways of des-γ-carboxyprothrombin in the progression of hepatocellular carcinoma. Can Invest. 2016;20;34(9):459–64. https://doi.org/10.1080/07357907.2016.1227445
- Yu R, Xiang X, Tan Z, Zhou Y, Wang H, Deng G. Efficacy of PIVKA-II in prediction and early detection of hepatocellular carcinoma: a nested case-control study in Chinese patients. Sci Rep. 2016;6(9):35050. https://doi.org/10.1038/srep35050
- Saitta C, Raffa G, Alibrandi A, Brancatelli S, Lombardo D, Tripodi G, et al. PIVKA-II is a useful tool for diagnostic characterization of ultrasound-detected liver nodules in cirrhotic patients. Medicine. 2017;96(26):e7266. https://doi.org/10.1097/MD.0000000000007266
- Huang S, Jiang F, Wang Y, Yu Y, Ren S, Wang X, et al. Diagnostic performance of tumor markers AFP and PIVKA-II in Chinese hepatocellular carcinoma patients. Tumor Biol. 2017;39(6):1010428317705763. https://doi.org/10.1177/1010428317705763
- Horn PS, Pesce AJ. Reference intervals: an update. Clin Chim Acta. 2003;334(1-2):5–23. https://doi.org/10.1016/S0009-8981(03)00133-5
- Fujita K, Kinukawa H, Ohno K, Ito Y, Saegusa H, Yoshimura T. Development and evaluation of analytical performance of a fully automated chemiluminescent immunoassay for protein induced by vitamin K absence or antagonist II. Clin Biochem. 2015;48(18):1330–6. https://doi.org/10.1016/j.clinbiochem.2015.07.023
- Gandham G, Jayamohanan H, Ponnada B, Kumar A, Keechilat P. Correlation of serum PIVKA-II and AFP level with portal vein tumor thrombus and BCLC stage in newly diagnosed hepatocellular carcinoma patients. J Clin Oncol. 38(15 suppl): e16608. https://doi.org/10.1200/JCO.2020.38.15_suppl.e16608
- Kim MJ, Bae KW, Seo PJ, Jeong IK, Kim JH, Lee BH, et al. Optimal cut-off value of PIVKA-II for diagnosis of hepatocellular carcinoma--using ROC curve. Korean J Gastroenterol. 2006;12(3):404–11.
- Viggiani V, Palombi S, Gennarini G, D’Ettorre G, De Vito C, Angeloni A, et al. Protein induced by vitamin K absence or antagonist-II (PIVKA-II) specifically increased in Italian hepatocellular carcinoma patients. Scand J Gastroenterol. 2016;51(10):1257–62. https://doi.org/10.1080/00365521.2016.1183705
Keywords: Hepatocellular carcinoma, Alpha Feto Protein, PIVKA-II, Reference interval, Cut-off value, Healthy controls
Publication History
Received: July 08, 2021
Revised: August 26, 2021 Revised: November 11, 2021
Accepted: December 12, 2021
Published: December 25, 2021
Authors
Faryal Husnain
Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan.
M Dilawar Khan
Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan.
Omar Rasheed Chughtai
Department of Histopathology, Chughtai Institute of Pathology, Lahore, Pakistan.
Akhtar Sohail Chughtai
Chief Executive, Chughtai Institute of Pathology, Lahore, Pakistan.
Shakeel Ashraf
Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan.
Ahmed Yar
Department of Chemical Pathology, Chughtai Institute of Pathology, Lahore, Pakistan.
