Original Article
Volume: 37 | Issue: 4 | Published: Dec 25, 2021 | Pages: 240 - 247 | DOI: 10.51441/BioMedica/5-350
Anti-Mullerian hormone and associated pregnancy outcomes in females with polycystic ovary syndrome undergoing In vitro fertilization-embryo transfer
Authors: Jiacheng Du , Yuping Cao ,
Article Info
Authors
Jiacheng Du
Reproductive Medicine Center, Second People's Hospital of Jingmen City, Hubei Province, 448000, China
Yuping Cao
Reproductive Medicine Center, Second People’s Hospital of Jingmen City, Jingmen City, China.
Publication History
Received: August 02, 2021
Accepted: December 08, 2021
Published: December 25, 2021
Abstract
Background and Objective: Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among reproductive-aged women worldwide. Pregnancy in these women is highly affected by serum levels of Anti-Mullerian hormone (AMH). This study aimed to determine the association of serum AMH levels with pregnancy outcomes in females with polycystic ovary syndrome (PCOS) undergoing in vitro fertilization-embryo transfer (IVF-ET)
Methods: A total of 200 PCOS patients undergoing IVF-ET in the Reproductive Medicine Center, Second People's Hospital of Jingmen, China were included. The patients were divided into two groups, A and B, based on their AMH levels. Serum sex hormones levels and pregnancy outcomes in terms of fertilization, cleavage, implantation, high quality embryo and biochemical and clinical pregnancy rates were compared.
Results: Mean age of the patients was 28.74±2.07 years in group A and 29.74±2.23 years in group B. Participants in group A had lower serum levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) compared to group B. Also, better pregnancy outcomes and lower frequency of pregnancy complications were seen in the participants of group A (P<0.05). AMH serum levels showed high specificity and sensitivity in predicting IVF-ET clinical pregnancy in PCOS patients.
Conclusion: Serum AMH levels in females with PCOS predict improved sex hormone profile and clinical pregnancy outcomes after IVF-ET
Keywords: Anti-Mullerian hormone; polycystic ovary syndrome; in vitro fertilization-embryo transfer; sex hormone levels; pregnancy outcome
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(4):240-247
ORIGINAL ARTICLE
Anti-Mullerian hormone and associated pregnancy outcomes in females with polycystic ovary syndrome undergoing in vitro fertilization-embryo transfer
Jiacheng Du1, Yuping Cao1*
Received: 02 August 2021 Revised date: 16 November 2021 Accepted: 08 December 2021
Correspondence to: Yuping Cao
*Reproductive Medicine Center, Second People’s Hospital of Jingmen City, Jingmen City, China.
Email: cyping_29@outlook.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among reproductive-aged women worldwide. Pregnancy in these women is highly affected by serum levels of Anti-Mullerian hormone (AMH). This study aimed to determine the association of serum AMH levels with pregnancy outcomes in females with PCOS undergoing in vitro fertilization-embryo transfer (IVF-ET)
Methods:
A total of 200 PCOS patients undergoing IVF-ET in the Reproductive Medicine Center, Second People’s Hospital of Jingmen, China were included. The patients were divided into two groups, A and B, based on their AMH levels. Serum sex hormones levels and pregnancy outcomes in terms of fertilization, cleavage, implantation, high quality embryo, and biochemical and clinical pregnancy rates were compared.
Results:
Mean age of the patients was 28.74 ± 2.07 years in group A and 29.74 ± 2.23 years in group B. Participants in group A had lower serum levels of follicle stimulating hormone, luteinizing hormone, and testosterone compared to group B. Also, better pregnancy outcomes and lower frequency of pregnancy complications were seen in the participants of group A (p < 0.05). AMH serum levels showed high specificity and sensitivity in predicting IVF-ET clinical pregnancy in PCOS patients.
Conclusion:
Serum AMH levels in females with PCOS predict improved sex hormones profile and clinical pregnancy outcomes after IVF-ET
Keywords:
Anti-Mullerian hormone, polycystic ovary syndrome, in vitro fertilization-embryo transfer, sex hormone levels, pregnancy outcome.
Introduction
Polycystic ovary syndrome (PCOS) is a relatively common endocrine and metabolic disorder in females of gestational age, characterized by symptoms, such as menstrual abnormalities, hirsutism, infertility, and acne.1 The clinical incidence of PCOS is relatively high affecting 5% to 10% women of gestational age.2 At present, the pathogenesis of PCOS is not completely understood in clinical practice, and there is no radical cure plan. The clinical treatment is mainly symptomatic, such as improving symptoms, completing fertility, promoting health, and improving quality of life.3 The infertility symptoms of PCOS patients are mainly due to their hyperandrogenemia, hyperinsulinemia and other diseases. The treatment is mainly oral anti-androgen active contraceptives or insulin sensitizers.4 However, some patients have drug ineffectiveness and long-term infertility, and therefore in vitro fertilization-embryo transfer (IVF-ET) is needed for fertility treatment.5 IVF-ET mainly achieves conception through controlled superovulation, in vitro fertilization and transplantation after egg retrieval, but some patients have poor pregnancy outcomes.6 At present, there is still a lack of clinically effective items to evaluate the pregnancy status and pregnancy outcome of patients with PCOS after IVF-ET.
Anti-Mullerian hormone (AMH) is member of the Transforming growth factor beta (TGF-β) superfamily, and its expression level reflects ovarian reserve and female reproductive ability to a certain extent, which is, the higher the expression of AMH, the more egg stock.7,8 The Expression of AMH in PCOS is about five times more than that of healthy ovulating women.9 AMH can also be used to evaluate the patient’s response to controlled ovarian stimulation during assisted reproduction therapy and predicts the number of oocytes.10 This study was conducted to determine the serum levels of AMH in PCOS patients undergoing IVF-ET in association with their pregnancy outcome.
Methods
This study was conducted in the Reproductive Medicine Center, Second People’s Hospital of Jingmen, China from January 2016 to January 2020 after taking approval from the Institutional Medical Ethics Committee. A total of 200 PCOS patients aged 25 to 36 years old undergoing IVF-ET were recruited. Patients fulfilling the criteria as (1) clinically diagnosed with PCOS; (2) undergoing IVF-ET; (3) complete clinical data and cooperation during treatment and follow-up, were included. While the patients with congenital immunodeficiency, severe infections, mental diseases, malignant tumors or severe cardiovascular and cerebrovascular diseases were excluded. A written informed consent was taken from all the study participants. The patients were divided into two groups: A (90) and B (110) based on their AMH levels. The serum levels of AMH in group A patients was less than 6.99 ng/ml, and it was greater or equal to 6.99 ng/ml in patients of group B. Participants were also distributed in group C and D according to the clinical outcomes of pregnancy.
AMH serum level detection
About 3 ml of fasting venous blood from the patients of both groups was drawn during their menstrual cycle. Blood samples were centrifuged at about 1,000-2,000 g for 10 minutes at 4°C. Enzyme-linked immunoassay (ELISA) was used to detect AMH levels. Standard was diluted with 0.05 M PH9 carbonate coating buffer to a protein content of 5 µg/ ml. Applied 100 µl to the reaction well of each polystyrene plate, incubated for 24 hours at 4°C. On day 2, discarded the reagent from the well and washing was done by washing buffer three times. (Referred to as washing, the same below). Then, applied 100 µl of diluted sample and incubated at 37°C for 60 minutes followed by washing. (At the same time, set blank wells and control wells). In each reaction well, added 0.1 ml of freshly diluted enzyme-labeled antibody, incubated at 37°C for 1 hour, washed, then added 0.1 ml of temporarily prepared Tetramethylbenzidine (TMB) substrate solution, 37°C for 30 minutes, and then added 0.05 ml of 2 M sulfuric acid. On the ELISA tester, zero adjusted the blank control well at 450 nm and then measured the optical density (O.D) value of each well to calculate the concentration. All ELISA test reagents were purchased from Shanghai Enzyme-Linked Biotechnology Co., Ltd.
Sex hormones detection
About 5 ml of fasting venous blood was drawn from all patients during their menstrual cycle, and follicle stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2), progesterone (P), testosterone (T), and prolactin (PRL) levels were determined by using ORG 300 automatic immunoassay analyzer (Germany ORGENTEC company). The operation was performed in strict accordance with the instrument’s protocols.
IVF-ET treatment method
Three weeks after patients taking oral contraceptives, triptorelin (Ferring GmbH, X20010072) pituitary downregulation, gonadotropin was used to stimulate ovulation, when there were three dominant follicles >18 mm, followed by Human chorionic gonadotropin (HCG) injection on the same day (Ma’anshan Fengyuan Pharmaceutical, H34023361) 6 k ~ 1 w U, 36 hours later, puncture egg was retrieved, and 72 hours after egg retrieval, embryo was transferred.
Serum levels of FSH, LH, E2, P, T, and PRL hormones, pregnancy outcomes including fertilization, cleavage, implantation, high-quality embryo, biochemical (HCG > 25 U/l in blood or urine) or clinical pregnancy rate, quality of embryos starting from ordinary fertilization, number of embryos 3 days after fertilization (6 to 10 embryos with a fragmentation degree of <15%), early miscarriage (spontaneous abortion in the first 12 weeks of pregnancy) and pregnancy complications were determined in participants of both groups.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences v 20. Mean and the standard deviation were used for quantitative variables. Chi-square test was applied to check the statistical significance and nonparametric test was used for comparison between the and receiver operating characteristic (ROC) curve was used to analyze the predictive value of AMH level in IVF-ET clinical pregnancy in PCOS patients. p < 0.05 was considered as statistically significant. GradpadPrism7.0 software package was used for mapping.
Results
A total of 200 participants were selected in this study. Mean age of the patients in group A was 28.74 ± 2.07 years while it was 29.74 ± 2.23 years in group D. Statistically, no significant difference in general information such as age, BMI, age at menarche, and infertility time was seen (p > 0.05). The patients were followed up for survival (Table 1; Figure 1).
Table 1. Comparison of general information of patients in the two groups.
| Groups | Group A (n = 90) | Group B (n = 110) | T value | p value |
|---|---|---|---|---|
| FSH (U/l) | 5.53 ± 1.26 | 7.93 ± 1.35 | 6.425 | 0.001 |
| LH (U/l) | 6.14 ± 0.83 | 8.21 ± 1.27 | 6.372 | 0.001 |
| E2 (pmol/l) | 128.73 ± 5.42 | 130.26 ± 5.49 | 0.586 | 0.524 |
| P (nmol/l) | 2.65 ± 1.04 | 2.81 ± 1.18 | 0.539 | 0.516 |
| T (nmol/l) | 1.47 ± 0.28 | 2.53 ± 0.36 | 6.197 | 0.001 |
| PRL (nmol/l) | 0.92 ± 0.35 | 0.86 ± 0.31 | 0.632 | 0.423 |
a p > 0.05 compared with group A.
b p > 0.05 compared with group C.
Figure 1. A combined diagram of the process.
Serum levels of FSH, LH, and T in group A were significantly lower than group B (p < 0.05), while E2, P, and PRL serum levels in group A were not significantly different (p > 0.05). The ovarian function of participants in the low-level AMH group(A) was significantly better than that in the high-level AMH group(B) (Table 2 and Figure 2).
Compared with patients in group B, the implantation, high-quality embryo, biochemical pregnancy, and clinical pregnancy rates in group A were significantly higher (p < 0.05). However, no significant difference in the fertilization and cleavage rate of patients were found between both groups (p > 0.05). The pregnancy outcomes of patients in the group A were significantly better than those in the group B (Table 3 and Figure 3).
Frequency of early miscarriage, gestational hypertension, and gestational diabetes in group A was significantly lower than group B (p < 0.05). The rate of early miscarriage and pregnancy complications in the group A was significantly lower than that in the group B (Table 4 and Figure 4).
Mean serum levels of AMH was (6.52 ± 1.04) ng/ml and (7.49 ± 1.12) ng/ml in the participants of group C and group D, respectively. The ROC curve was applied to analyze predictive value of AMH level in IVF-ET clinical pregnancy in PCOS patients, and the results showed that it has high specificity (77.52%) and sensitivity (81.46%) (Figure 5).
Table 2. Comparison of mean sex hormone levels between the two groups (x ± s).
| Groups | Group A (n = 90) | Group B (n = 110) | T value | p value |
|---|---|---|---|---|
| FSH (U/l) | 5.53 ± 1.26 | 7.93 ± 1.35 | 6.425 | 0.001 |
| LH (U/l) | 6.14 ± 0.83 | 8.21 ± 1.27 | 6.372 | 0.001 |
| E2 (pmol/l) | 128.73 ± 5.42 | 130.26 ± 5.49 | 0.586 | 0.524 |
| P (nmol/l) | 2.65 ± 1.04 | 2.81 ± 1.18 | 0.539 | 0.516 |
| T (nmol/l) | 1.47 ± 0.28 | 2.53 ± 0.36 | 6.197 | 0.001 |
| PRL (nmol/l) | 0.92 ± 0.35 | 0.86 ± 0.31 | 0.632 | 0.423 |
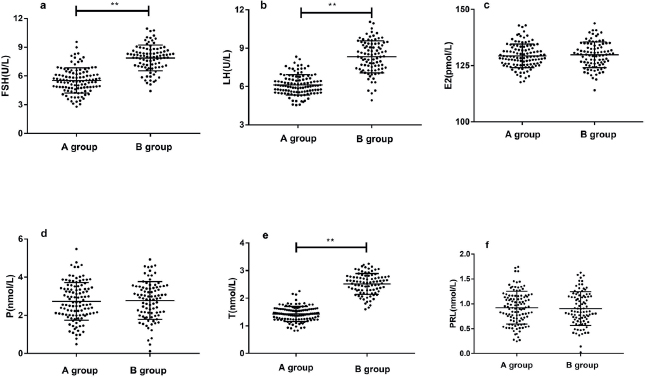
Figure 2. Image comparison of sex hormone levels in patients of both groups (A and B). (A) follicle stimulating hormone (FSH); (b) luteinizing hormone (LH); (c) estradiol (E2); (d) progesterone (P); (e) is testosterone (T); (f) prolactin (PRL); ** means p < 0.001.
Table 3. Comparison of pregnancy outcomes of patients between the two groups (n, %).
| Groups | Group A (n = 90) | Group B (n = 110) | X2 value | p value |
|---|---|---|---|---|
| Fertilization rate (%) | 80.00 (73/90) | 78.18 (86/110) | 0.574 | 0.526 |
| Cleavage rate (%) | 98.89 (89/90) | 98.18 (108/110) | 0.639 | 0.438 |
| Implantation rate (%) | 41.11 (37/90) | 20.91 (23/110) | 6.541 | 0.001 |
| High quality embryo rate (%) | 52.22 (47/90) | 40.91 (45/110) | 6.325 | 0.001 |
| Biochemical pregnancy rate (%) | 64.44 (58/90) | 51.82 (57/110) | 6.293 | 0.001 |
| Clinical pregnancy rate (%) | 57.78 (52/90) | 32.73 (36/110) | 6.741 | 0.001 |
Discussion
PCOS being a complex disease caused by metabolic and endocrine disorders, mainly affects women of childbearing age.11 PCOS patients often have symptoms such as hyperandrogenemia, hyperinsulinemia, ovulationdysfunction or even anovulation, so patients often are accompanied by infertility symptoms.12 IVF-ET therapy is often used clinically for infertile PCOS patients with ineffective drug therapy. However, there are certain differences in the pregnancy outcomes of PCOS infertile patients with different conditions after IVF-ET treatment.13 Both AMH and sex hormone levels effectively depict ovarian reserve in women of gestational age, and changes in its expression are of great significance in some infertility diseases. 14
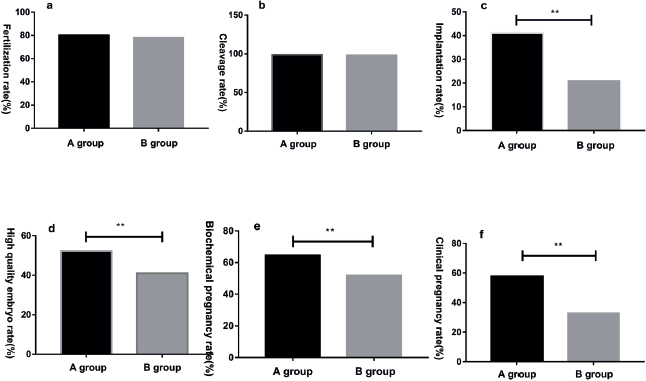
Figure 3. Image comparison of pregnancy outcomes in patients of both groups (A and B); (a) fertilization rate; (b) cleavage rate; (c) implantation rate; (d) high quality embryo rate; (e) biochemical pregnancy rate; (f) clinical pregnancy rate; ** means p < 0.001.
Table 4. Comparison of pregnancy related complications in both groups (n, %).
| Groups | Group A (n = 90) | Group B (n = 110) | X2 value | p value |
|---|---|---|---|---|
| Early abortion rate (%) | 6.67 (6/90) | 19.09 (21/110) | 6.315 | 0.001 |
| Pregnancy induced hypertension (%) | 13.33 (12/90) | 31.82 (35/110) | 6.762 | 0.001 |
| Gestational diabetes mellitus (%) | 3.33 (3/90) | 17.27 (19/110) | 6.439 | 0.001 |
AMH is one of the important members of the TGF-β superfamily, and it mainly plays a physiological role through AMH receptor II.15 Serum AMH is one of the important indicators for diagnosing PCOS patients. AMH levels in PCOS patients are about two to three times higher than that of normal ovulating women, therefore its expression helps in diagnosing PCOS.16 In addition, the serum AMH level can also be used in the assessment of ovarian reserve. Poor ovarian reserve may lead to menopause and infertility in women of gestational age.17 The current study showed significantly lower levels of T, FSH and LH in group A while comparing to group B. The pathological increase of their levels in latter may indicate women’s poor ovarian function and ovarian regression, to certain extent, that may have a negative impact on normal pregnancy in women of gestational age. Ishii et al.18 found that high expression levels of T in turn promote the secretion of AMH and cause incomplete follicular development. Increased expression levels of AMH, FSH, and LH are important as this may cause hyperandrogenemia in the body, and further damage to follicular function.19 Current study also shows significantly higher serum levels of FSH, LH, and T in group B participants which indicate poor frontal ovarian function in these patients. However, there was no significant difference in E2, P, and PRL levels between groups A and B in terms of the pregnancy outcome, implantation, high-quality embryo, biochemical pregnancy, and clinical pregnancy rates were significantly better in the participants of group A. Bedenk et al.20 reported that the pathological increase of serum AMH level not only affects the ovarian function of pregnant women, but also damages the normal ovarian environment, resulting in poor oocyte and embryos quality, affecting normal pregnancy. Detti et al.21 related the pathological expression of serum AMH, FSH, LH, and T levels with the expression of cytokines related to endometrial morphology and endometrial receptivity, resulting in higher endometrial volume. Also, the receptivity is reduced, affecting normal pregnancy.21 Therefore, in the current study, the pregnancy and embryo quality of group A patients were closely related to their lower serum FSH, LH, T, and AMH expression.

Figure 4. Image comparison of early abortion rate and pregnancy complications of patients in of both groups (A and B); (a) early abortion rate; (b) pregnancy induced hypertension; (c) gestational diabetes mellitus; ** means p < 0.001.
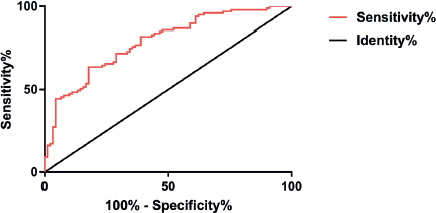
Figure 5. ROC curve of AMH levels predicting IVF-ET in clinical pregnancy in PCOS patients ROC curve analysis shows that the area below the curve is 0.783, 95% of CI is 0.586 to 0.826, the specificity is 77.52%, the sensitivity is 81.46%, the Youden index is 53.26, and the optimal cut-point is 7.149.
High androgen status in PCOS patients affects the insulin metabolism of related cytokines in the uterus, thus having a certain impact on the normal development of the placenta, causing the hormones secreted by the placenta to enhance insulin resistance. Therefore, PCOS patients are prone to have diabetes during pregnancy.22,23 According to another study, high androgen levels in PCOS patients cause increased secretion of vascular smooth muscle cells, resulting in narrowing of the vascular lumen, increased blood flow resistance, poor vascular endothelial function, and eventually the development of pregnancy-induced hypertension.24 In this study, compared with patients in group B, the incidence of gestational diabetes and hypertension in pregnancy in group A was significantly lower, which may be due to the low serum AMH level. In terms of the early abortion rate, it was seen significantly better in the participants of group A, which was more related to their endometrial morphology and function. Therefore, this study found low serum AMH levels improve the androgen status and intrauterine environment of PCOS patients, and reduce the rate of early pregnancy miscarriage and related complications during IVF-ET. In the present study, ROC curve showed the high specificity and sensitivity of AMH serum levels in IVF-ET clinical pregnancy in PCOS patients, which is consistent with the findings of Melado et al. 25 Clinical interventions can be taken in advance for PCOS patients with high serum AMH levels when undergoing IVF-ET to improve their pregnancy outcome.
Conclusion
Females with PCOS have lower serum AMH levels and better sex hormones profile that relates to clinical pregnancy outcomes during IVF-ET. Serum AMH levels reliably predict IVF-ET clinical pregnancy in these patients. Therefore, patients with high levels of AMH should be offered interventions in advance to improve outcomes of pregnancy during IVF-ET.
Limitations of the Study
The present study investigated only the clinical pregnancy and early miscarriage rate of patients, while the patient’s late pregnancy process or the health of the newborn was not studied. The trial period should be extended in future studies. Also, there is a need to comprehensively explore the relationship between AMH level and the whole process of IVF-ET pregnancy in PCOS patients.
Acknowledgement
The authors are thankful to all the staff and clinicians of the Reproductive Medicine Center, Second People’s Hospital of Jingmen City, Hubei China for their technical and logistic support for execution of this study.
List of Abbreviations
| AMH | Anti-Mullerian hormone |
| E2 | Estradiol |
| FSH | Follicle stimulating hormone |
| HCG | Human chorionic gonadotropin |
| IVF-ET | In vitro fertilization-embryo transfer |
| LH | Luteinizing hormone |
| O.D | Optical Density |
| P | Progesterone |
| PCOS | Polycystic ovary syndrome |
| PRL | Prolactin |
| ROC | Receiver Operating Characteristic |
| T | Testosterone |
| TGF | Transforming growth factor beta |
| TMB | Tetramethylbenzidine |
Conflict of interests
None to declare
Grant support and financial disclosure
None to disclose.
Ethical approval
The present study was approved by Second People’s Hospital of Jingmen City Medical Ethics Committee (Hubei, China). (Approval number:R32A11).
Authors’ contribution
JD, YC: Conception of study, acquisition and analysis of data, intellectual input and drafting the manuscript.
All Authors: Approval of the final version of the manuscript to be published.
Authors’ details
Jiacheng Du1, Yuping Cao1
- Reproductive Medicine Center, Second People’s Hospital of Jingmen City, Jingmen City, China
References
- Kumari R, Kanika J, Debasis D. Comparison of clinical outcomes in clomiphene citrate resistant infertile polycystic ovarian syndrome women after treatment with laparoscopic ovarian drilling (LOD) versus gonadotropins. Int J Reprod Contracept Obstet Gynecol. 2018;7(4):576–81. https://doi.org/10.18203/2320-1770.ijrcog20180175
- Katsigianni M, Karageorgiou V, Lambrinoudaki I, Siristatidis C. Maternal polycystic ovarian syndrome in autism spectrum disorder: a systematic review and meta-analysis. Mol Psychiatr. 2019;24(12):1787–97. https://doi.org/10.1038/s41380-019-0398-0
- Tan IF, Lim AJ, Indran IR, Kramer MS, Yong EL. Reproductive outcomes of women with polycystic ovarian syndrome following in-vitro fertilization - a meta-analysis and systematic review. Fertil Reprod. 2019;01(4):193–201. https://doi.org/10.1142/S2661318219500221
- Chehin MB, Fraietta R, Lorenzon AR, Bonetti TC, Motta EL. The insulin signaling pathway is dysregulated in cumulus cells from obese, infertile women with polycystic ovarian syndrome with an absence of clinical insulin resistance. Ther Adv Reprod Health. 2020;14(3):2633494120906866. https://doi.org/10.1177/2633494120906866
- Estes JL, Pereira N, Lekovich J, Spandorfer SD. Perinatal outcomes of singletons following in vitro fertilization in women with polycystic ovarian syndrome [22G]. Obst Gyn. 2016;127(4):64S–5. https://doi.org/10.1097/01.AOG.0000483906.07129.f6
- Chen YH, Wang Q, Zhang YN, Han X, Li DH, Zhang CL. Cumulative live birth and surplus embryo incidence after frozen-thaw cycles in PCOS: how many oocytes do we need? J Assist Reprod Genet. 2017;34(9):1153–9. https://doi.org/10.1007/s10815-017-0959-6
- Moini A, Pirjani R, Rabiei M, Nurzadeh M, Sepidarkish M, Hosseini R, et al. Can delivery mode influence future ovarian reserve? Anti-Mullerian hormone levels and antral follicle count following cesarean section: a prospective cohort study. J Ovarian Res. 2019;12(1):1–7. https://doi.org/10.1186/s13048-019-0551-z
- Hafizi L, Behrouznia A, Amirian M, Baradaran M, Pourhoseini SA. Evaluation of anti-mullerian hormone predictive value and antral follicle count in the success rate of ovarian drilling in polycystic ovary syndrome. Shiraz E Med J. 2020;21(6). https://doi.org/10.5812/semj.92162.
- Chiofalo F, Ciuoli C, Formichi C, Selmi F, Forleo R, Neri O, et al. Bariatric surgery reduces serum anti-mullerian hormone levels in obese women with and without polycystic ovarian syndrome. Obes Surg. 2017;27(7):1750–4. https://doi.org/10.1007/s11695-016-2528-y
- Köninger A, Kampmeier A, Mach P, Schmidt B, Strowitzki T, Kimmig R, et al. Tight interplay in early pregnancy between follistatin and anti-mullerian hormone in women with polycystic ovarian syndrome (PCOS). Arch Gynecol Obstet. 2018;297(5):1307–16. https://doi.org/10.1007/s00404-018-4718-4
- Al-Gareeb AI, Abd Al-Amieer WS, Alkuraishy HM, Al-Mayahi TJ. Effect of body weight on serum homocysteine level in patients with polycystic ovarian syndrome: a case control study. Int J Reprod Biomed. 2016;14(2):81.
- Kim J, Mersereau JE, Khankari N, Bradshaw PT, McCullough LE, Cleveland R, et al. Polycystic ovarian syndrome (PCOS), related symptoms/sequelae, and breast cancer risk in a population-based case-control study. Cancer Cause Control. 2016;27(3):403–14. https://doi.org/10.1007/s10552-016-0716-7
- Pereira N, Lekovich J, Rosenwaks Z, Spandorfer SD. Do serum anti-Mullerian hormone levels correlate with pregnancy outcomes in patients with diminished ovarian reserve undergoing in vitro fertilization? Fertil Steril. 2015;104(3):e93. https://doi.org/10.1016/j.fertnstert.2015.07.286
- Simões-Pereira J, Nunes J, Aguiar A, Sousa S, Rodrigues C, Matias JS, et al. Influence of body mass index in anti- Müllerian hormone levels in 951 non-polycystic ovarian syndrome women followed at a reproductive medicine unit. Endocrine. 2018;61(1):144–8. https://doi.org/10.1007/s12020-018-1555-y
- Kocaay P, Siklar Z, Buyukfirat S, Berberoglu M. The diagnostic value of anti-mullerian hormone in early post menarche adolescent girls with polycystic ovarian syndrome. J Pediatr Adol Gynec. 2018;31(4):362–6. https://doi.org/10.1016/j.jpag.2018.02.126
- Vagios S, Sacha CR, Hsu JY, Dimitriadis I, Bormann CL, James KE, et al. Can anti-mullerian hormone (AMH) levels predict response to ovulation induction treatments in women with polycystic ovarian syndrome (PCOS)? Fertil Steril. 2019;112(3):e391–2. https://doi.org/10.1016/j.fertnstert.2019.07.1118
- de Loos AD, Hund M, Buck K, Meun C, Sillman J, Laven JS. Establishing an anti-müllerian hormone (AMH) cut-off to determine polycystic ovarian morphology (PCOM) supporting diagnosis of polycystic ovarian syndrome (PCOS): the aphrodite study. Fertil Steril. 2019;12(3):e391. https://doi.org/10.1016/j.fertnstert.2019.07.1116
- Ishii R, Tachibana N, Okawa R, Enomoto M, Asami M, Toriumi R, et al. Different anti-Mallerian hormone (AMH) levels respond to distinct ovarian stimulation methods in assisted reproductive technology (ART): clues to better ART outcomes. Reprod Med Biol. 2019;18(3):263–72. https://doi.org/10.1002/rmb2.12270
- Cai WY, Gao JS, Luo X, Ma HL, Ge H, Liu N, et al. Effects of metabolicabnormalities, hyperandrogenemiaandclomiphene on liver function parameters among Chinese women with polycystic ovary syndrome: results from a randomized controlled trial. J Endocrinol Invest. 2019;42(5):549–55. https://doi.org/10.1007/s40618-018-0953-6
- Bedenk J, Vrtačnik-Bokal E, Virant-Klun I. The role of anti- Müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet. 2020;37(1):89–100. https://doi.org/10.1007/s10815-019-01622-7
- Detti L, Williams LJ, Osborne SE, Fletcher NM, Saed GM. Elevated serum anti-Mullerian hormone (AMH) stalls ovarian follicle development by downregulating FSH- and LH-receptors and Inhibin-B production. Fertil Steril. 2015;104(3):e6. https://doi.org/10.1016/j.fertnstert.2015.07.019
- Li G, Huang W, Zhang L, Tian Z, Zheng W, Wang T, et al. A prospective cohort study of early-pregnancy risk factors for gestational diabetes in women with polycystic ovarian syndrome. Diabetes Metab Res. 2018;34(5):e3003. https://doi.org/10.1002/dmrr.3003
- Cree-Green M, Cai N, Thurston JE, Coe GV, Newnes L, Garcia- Reyes Y, et al. Using simple clinical measures to predict insulin resistance or hyperglycemia in girls with polycystic ovarian syndrome. Pediatr Diabetes. 2018;19(8):1370–8. https://doi.org/10.1111/pedi.12778
- Pergialiotis V, Trakakis E, Chrelias C, Papantoniou N, Hatziagelaki E. The impact of mild hypercholesterolemia on glycemic and hormonal profiles, menstrual characteristics and the ovarian morphology of women with polycystic ovarian syndrome. Horm Mol Biol Clin. 2018;34(3):1–7. https://doi.org/10.1515/hmbci-2018-0002
- Melado Vidales L, Fernández-Nistal A, Martínez Fernández V, Verdú Merino V, Bruna Catalán I, Bajo Arenas JM. Anti-Müllerian hormone levels to predict oocyte maturity and embryo quality during controlled ovarian hyperstimulation. Minerva Ginecol. 2017;69(3):225–32. https://doi.org/10.23736/S0026-4784.16.03958-7
