Original Article
Volume: 37 | Issue: 3 | Published: Sep 25, 2021 | Pages: 191 - 198 | DOI: 10.51441/BioMedica/5-512
Differential expression of markers of oxidative stress and apoptosis in relation to serum ferritin levels in patients with pre-eclampsia
Authors: Jianying Yan , Jie Dong , Xiaoqian Lin , Lichun Chen , Zhuanji Fang , Qing Han , Qinjian Zhang , Lingling Jiang , Xia Xu , Xu Lin
Article Info
Authors
Jianying Yan
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University-China.
Jie Dong
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China
Xiaoqian Lin
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China
Lichun Chen
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China
Zhuanji Fang
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China.
Qing Han
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China.
Qinjian Zhang
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China.
Lingling Jiang
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China.
Xia Xu
Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou-China.
Xu Lin
Key Lab of Ministry of Edu for Gastrointestinal Cancer, School of Basic Med Sci, Fujian Medical University, Fuzhou-China.
Publication History
Received: April 17, 2021
Accepted: September 14, 2021
Published: September 25, 2021
Abstract
Background and Objective: Pre-eclampsia (PE) is a hypertensive gestational disease appearing during second trimester of pregnancy. Free radicals are released by the placenta in this condition that may cause oxidative damage. This study was designed to determine the serum ferritin (SF) levels in maternal blood, fetal umbilical cord blood and placenta and the changes associated with oxidative stress as well as cell apoptosis to understand the pathogenesis of PE.
Methods: This cross-sectional analytical study recruited 60 pregnant females with severe PE and assigned into early and late onset PE groups. Another n = 60 cases of normal pregnant females with similar gestational weeks were selected in the control group. Maternal serum and fetal umbilical cord blood ferritin levels were determined by automatic biochemical immunoassay system. Reverse transcription real-time fluorescence, Western blot and colorimetry were used to determine mRNA expression levels of ferritin and ferritin heavy chain, relative expression of ferritin and superoxide dismutase, malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) levels, respectively.
Results: Mean age was 30.89 ± 5.65 and 31.79 ± 5.06 years in early and late onset PE groups respectively. Serum uric acid and creatinine levels of both PE groups were significantly higher than the normal pregnant females. SF levels were also higher in the participants of PE groups. However, the mRNA and ferritin protein levels in placental tissue were significantly lower in PE groups while comparing to controls. The cleaved caspase-3 protein, GSH-Px and MDA levels were significantly higher in both PE groups (p < 0.05).
Conclusion: The alterations in factors related to oxidative stress and cell apoptosis in placental tissue may be helpful to understand the pathogenesis of PE and may provide potential biomarkers for the diagnosis of PE.
Keywords: Ferritin, pre-eclampsia, oxidative stress, apoptosis, pregnancy, biomarkers.
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(3):191-198
ORIGINAL ARTICLE
Differential expression of markers of oxidative stress and apoptosis in relation to serum ferritin levels in patients with pre-eclampsia
Jianying Yan1*, Jie Dong1, Xiaoqian Lin1, Lichun Chen1, Zhuanji Fang1, Qing Han1, Qinjian Zhang1, Lingling Jiang1, Xia Xu1, Xu Lin2,3
Received: 17 April 2021 Revised date: 28 August 2021 Accepted: 14 September 2021
Correspondence to: Jianying Yan
*Department of Obstetrics, Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, China.
Email: xinyzh2018@126.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Pre-eclampsia (PE) is a hypertensive gestational disease appearing during second trimester of pregnancy. Free radicals are released by the placenta in this condition that may cause oxidative damage. This study was designed to determine the serum ferritin (SF) levels in maternal blood, fetal umbilical cord blood and placenta and the changes associated with oxidative stress as well as cell apoptosis to understand the pathogenesis of PE.
Methods:
This cross-sectional analytical study recruited 60 pregnant females with severe PE and assigned into early and late onset PE groups. Another n = 60 cases of normal pregnant females with similar gestational weeks were selected in the control group. Maternal serum and fetal umbilical cord blood ferritin levels were determined by automatic biochemical immunoassay system. Reverse transcription real-time fluorescence, Western blot and colorimetry were used to determine mRNA expression levels of ferritin and ferritin heavy chain, relative expression of ferritin and superoxide dismutase, malondialdehyde (MDA), and glutathione peroxidase (GSH-Px) levels, respectively.
Results:
Mean age was 30.89 ± 5.65 and 31.79 ± 5.06 years in early and late onset PE groups respectively. Serum uric acid and creatinine levels of both PE groups were significantly higher than the normal pregnant females. SF levels were also higher in the participants of PE groups. However, the mRNA and ferritin protein levels in placental tissue were significantly lower in PE groups while comparing to controls. The cleaved caspase-3 protein, GSH-Px and MDA levels were significantly higher in both PE groups (p < 0.05).
Conclusion:
The alterations in factors related to oxidative stress and cell apoptosis in placental tissue may be helpful to understand the pathogenesis of PE and may provide potential biomarkers for the diagnosis of PE.
Keywords:
Ferritin, pre-eclampsia, oxidative stress, apoptosis, pregnancy, biomarkers.
Introduction
Pre-eclampsia (PE) and eclampsia are the main causes of perinatal maternal and fetal death.1 The overall incidence of PE and eclampsia accounts for 4.6% and 1.4% of the total pregnancies, respectively.2 In some developed regions (Eastern Mediterranean), the incidence of PE is only 1.0%, whereas in Africa and Brazil it accounts for 5.6% to 7.5% respectively.2,3 Hebei Province of China reports the incidence of mild and severe PE as 1.38% and 4.02% respectively based on the data of more than 30,000 cases.4 The number of perioperative deaths caused by PE accounts for 0.02% of the total pregnancies.5 In addition, the incidence of stroke, heart failure, and diabetes in pregnant females with PE is a predictive notion that cannot be considered pivotal till now.6 The standard of diagnosis of PE is based on the mean systolic and diastolic blood pressures after 20 weeks of pregnancy, dysfunctioning of maternal organs (like kidney, liver, blood, or brain) and/or limited fetal growth.7
Patients with PE usually show abnormal serum ferritin (SF) levels thereby it is considered as a potential biomarker for evaluating PE and pregnancy outcome.8 Ferritin is a macromolecular protein containing ferric ion. SF is also closely associated with hypertension, coronary artery, or cerebrovascular disease.9 Besides SF, there is a significant increase in lipid peroxide in patients with PE while a decrease in antioxidants may indicate the correlation between oxidative stress reactions and PE.10 Furthermore, placental cell apoptosis plays an essential role in the developing PE indicating an imbalance of cell apoptosis.11 Caspase-3, Bcl-2, and Bax and other cell apoptotic genes have been reported as differentially expressed proteins in PE.12,13,14
Therefore, this study was designed to determine the SF levels in maternal and fetal umbilical cord blood and placental tissue; moreover, serum caspase-3, Bcl-2, Bax, glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), and malondialdehyde (MDA) levels were compared in placental tissues of PE patients and healthy pregnant females. Furthermore, the neonatal outcomes in PE groups and normal controls were compared.
Methods
This cross-sectional analytical study was conducted at the Department of Obstetrics, Fujian Provincial Maternity and Child Health Hospital, Fujian Medical University, China from December 2017 to December 2018 after getting the approval from the institutional ethical committee. A total of n = 120 admitted pregnant females were included after taking written informed consent. Among them, n = 60 pregnant females diagnosed with PE were randomized equally (n = 30) in early-onset (gestational weeks at onset ≤ 34 weeks) and the late-onset PE (gestational weeks at onset > 34 weeks) groups. Another n = 60 normal pregnant females from 28 to 36 weeks of gestation (control group) were recruited.
PE was diagnosed based on the criteria recommended by the International Society for the Study of Hypertension in Pregnancy including the hypertensive females with albuminuria (>300 mg) after 20 weeks of pregnancy.15 Females with heart disease, essential hypertension, renal diseases, gestational diabetes mellitus, intrahepatic cholestasis of pregnancy, multiple pregnancies, fetal malformations, parturient or females with ruptured membranes or signs of clinical infection, and a mode of delivery other than cesarean section were excluded. Age, body mass index (BMI), serum uric acid (UA), and creatinine (Cr) levels and neonatal outcomes in both PE and normal control group were also determined.
After overnight fasting, blood samples (3 ml blood) were collected from each subject’s arm (8 hours after “lights-off” for photoperiodic control). The blood samples were centrifugated (3,000 g, 15 minutes) and then stored under -80°C. Blood (3 ml) was extracted from the umbilical cord vein instantly after delivery. Also, the placental tissue from the root of umbilical cord was collected after Cesarean section and cut into pieces of 1.0 × 1.0 × 1.0 cm under sterile condition; infarcted and calcified areas were avoided. The placental samples were rinsed with cold saline, wrapped with tin foil, and then put into 1.5 ml cryopreservation tube and stored under -80°C. Since the subjects in the control group were healthy pregnant females with no premature delivery, only serum sample was used for analysis.
Reverse transcription real-time fluorescence (RT-qPCR) analysis
The placental tissues were treated with TRIzol reagent to extract the total RNA in the tissues. The first chain of DNA was synthesized with RevertAid first strand cDNA synthesis kit. QuantiNova SyBr Green polymerase chain reaction (PCR) kit was used to perform PCR analysis. Reaction conditions consisted of pre-denaturation at 95°C for 1 minute, denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 30 seconds, running 40 cycles. Primers for ferritin gene: forward primer 5′- TGTACCTGCAGGCCTCCTACA -3′; reverse primer: 5′- GACGCCTTCCAGAGCCACAT-3′; probe primer: 5′- TACCTCTCTCTGGGCTTCTATTTCGACCGC -3′; Primers for ferritin heavy chain (FTH) gene: forward primer 5′-AACATGCTG AGAAACTGATGAAGCT-3′ ; reverse primer 5′- GTCATCAC AGTCTGGTTTCTTGATATC-3′; probe primer: 5′- AACCAAC GAGGTGGCCGAATCTTCC -3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as the internal control. The relative expression of mRNA was calculated by the 2ΔΔCt and there was no unit in the plotted columns.
Colorimetric methods to determine T-SOD and MDA levels
First, the placental tissue was washed 3-5 times with erythrocyte lysate, and then washed 3 times with phosphate buffer. Tissue homogenate (10%) was prepared by adding saline solution with a weight to volume ratio of 1:9. Bicinchoninic acid (BCA) kit was used to determine protein concentration. MDA was detected for tissue homogenate using a colorimetric assay kit, The supernatant was collected after the tissue homogenate was centrifuged at 4°C with 1,600 × g/minute for 10 minutes, and absorbance was reported at 532 nm. Water soluble tetrazolium-8 detection method was used to measure the levels of SOD. The supernatant was collected after the tissue homogenate was centrifuged at 4°C with 1,500 × g/minute for 5 minutes, and absorbance was recorded at 450 nm.
Western blot analysis
First, the placental tissue was washed 3-5 times with erythrocyte lysate and then washed with phosphate buffer for 3 times. Radioimmunoprecipitation assay buffer was used for lysis and then it was centrifuged at 4°C for 10 minutes (at 12,000 g speed). The protein concentration was determined with BCA protein assay kit (BiYunTian). The extracted proteins (40 μg) of each tissue sample were transferred to the polyvinylidene fluoride membrane (Millipore, Billerica, MA) after separating by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE). Skim milk (5%) was used to block the membrane at 25°C for 1 hour and incubated with primary antibody (1:1,000) overnight. After washing with phosphate-buffered saline, 0.1% Tween (PBST), incubation of the membrane was done at 25°C for 1 hour with secondary antibody conjugated with horseradish peroxidase followed by three washings. Enhanced chemiluminescence (BeyoECL Plus, 100 ml, BiYunTian) was used to detect the protein.
TUNEL assay
Placental samples were fixed overnight with 4% paraformaldehyde, except the samples collected from basal plate and the decidua to avoid the contamination of maternal cells. terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) immunohistochemical analysis was performed using TUNEL apoptotic assay kit (Roche, Cat. No. 11684795910, US) following the manufacturer’s instructions. The sections prepared in this experiment were washed with phosphate buffer saline (PBS) for 3 times. The slides were incubated in Triton X-100 solution (P0096-100 ml, BiYunTian) on ice for 2 minutes. The labelled mixture in terminal deoxytransferase enzyme buffer containing biotinylated deoxyuridine 5-triphosphate (dUTP) was added to the slides and incubated at 37°C for 60 minutes with 5% CO2. The slides were washed with PBS twice. The area around the sample was dried. According to the tissue size, the sections were added with diamidino-2-phenylindole (DAPI) (C1006, BiYunTian) and stood for 30 seconds. Then, the slides were washed with PBS three times. The presence of green fluorescence in cytoplasm was regarded as positive, while the absence of green fluorescence was negative. Leica inverted microscope was used in 400-fold high-power field of vision. Three non-overlapping fields were selected for each slide. The number of positive cells in 200 cells was counted in each field of vision. The apoptotic rate = number of apoptotic positive cells/total number of cells × 100%.
Statistical analysis
The data were analyzed by using Statistical Package for the Social Sciences version 22.0 software and GraphPad Prism Version 7.0. The continuous variables were measured as means ± SEM. Unpaired student’s t-test was used for comparison between the two groups while two-way analysis of variance was applied for three or more groups. p < 0.05 was set as statistically significant.
Results
This study included n = 120 participants and the mean age was 30.89 ± 5.65 and 31.79 ± 5.06 years in early and late onset PE group, respectively. High BMI was found in both PE groups (Table 1).
Regrading, the neonatal outcomes, body weight of newborns belonging to both PE groups and control group were 1.80, 2.84 and 3.57 kg respectively. Body weight and length of neonates belonging to PE groups were significantly smaller (Table 3).
Table 1. General information and blood pressure measurement in each groups (× ± seconds).
| Group | Cases | Age (years) | Gestational week | Pre-pregnancy BMI | BMI during pregnancy | Sytolic pressure (mmHg) | Diastolic pressure (mmHg) | MAP (mmHg) |
|---|---|---|---|---|---|---|---|---|
| Early onset control group | 30 | 30.10 ± 5.17 | 32.20 ± 0.88 | 19.98 ± 1.49 | 25.02 ± 3.06 | 114.03 ± 8.98 | 70.57 ± 6.16 | 85.06 ± 5.49 |
| Early onset PE group | 30 | 30.89 ± 5.65 | 33.08 ± 2.66 | 22.88 ± 3.55 | 28.13 ± 3.81 | 147.81 ± 18.40 | 99.67 ± 15.06 | 115.72 ± 15.24 |
| Late onset control group | 30 | 32.03 ± 4.79 | 39.17 ± 0.71 | 21.09 ± 2.48 | 26.63 ± 2.51 | 117.07 ± 7.56 | 75.20 ± 5.72 | 89.16 ± 5.01 |
| Late onset PE group | 30 | 31.79 ± 5.06 | 37.57 ± 1.24 | 26.45 ± 9.94 | 29.02 ± 3.90 | 138.46 ± 17.49 | 91.25 ± 10.86 | 106.99 ± 12.26 |
DP = Diastolic pressure, MAP = Mean arterial pressure.
The serum UA and Cr levels in both early and late-onset PE groups were approximately similar (p > 0.05). Though a significant variation in 24 hours proteinuria levels was seen in early-onset [1.67 (0.53-11.12)] as compared to the late-onset PE group [0.46 (0.26-7.79)] (Table 2).
Table 2. Renal function comparison between groups (× ± seconds).
| Group | Number of cases | UA (mmol/l) | Cr (mmol/l) | 24 hours proteinuria (g) |
|---|---|---|---|---|
| Early onset PE group | 30 | 4.59 ± 1.69a | 57.70 ± 17.27a | 1.67 (0.53-11.12)a |
| Late onset PE group | 30a | 4.02 ± 1.12a | 51.74 ± 19.83a | 0.46 (0.26-7.79)a |
| Control | 30 | 3.36 ± 0.70 | 44.64 ± 8.12 | - |
a Represents compared with control group (p < 0.05).
Table 3. Neonatal characteristics in PE and control groups.
| Neonatal features | Control group | Early onset PE group | Late onset PE group |
|---|---|---|---|
| Number of cases | 30 | 30 | 30 |
| Body weight (kg) | 3.57 ± 0.4 | 1.80 ± 0.69a | 2.84 ± 0.47a |
| Body length (cm) | 50.6 ± 1.5 | 41.8 ± 4.7a | 47.8 ± 2.2a |
| Gestational week at birth (weeks) | 39.2 ± 0.7 | 33.1 ± 2.7a | 37.6 ± 1.2a |
| Number of Neonatal Intensive Care Unit occupancy | 1 (0.03) | 21 (70.0)a | 8 (26.7)a |
| Days of NICU occupancy (days) | 0 | 16.8 ± 11.9a | 1.86 ± 1.68a |
| Premature delivery | 0 | 25 (83.3)a | 0 |
| Neonatal respiratory distress syndrome | 0 | 3 (0.1)a | 0 |
| Necrotizing enterocolitis | 0 | 1 (0.03)a | 0 |
| Hyperbilirubinemia | 6 (0.2) | 1 (0.03)a | 1 (0.03)a |
| Bronchopulmonary dysplasia | 0 | 1(0.03)a | 0 |
Levels of GSH-Px, T-SOD and MDA in placental tissues
The mean expression levels of GSH-Px and MDA of early onset (177.67 ± 65.04 U/mgprot; 49.93 ± 14.64 μmol/l) and late onset PE groups (201.57 ± 55.97 U/mgprot; 45.66 ± 18.93 μmol/l) were higher than the control group (95.50 ± 44.98 U/mgprot; 13.94 ± 7.73 μmol/l) (p < 0.05).
The mean expression levels of T-SOD were lower in early onset (1.03 ± 0.24) and late onset PE group (0.91 ± 0.21 units) than the late onset control group (1.33 ± 0.48 units) (p < 0.05), and both of the PE groups did not show significant difference for expression levels of GSH-Px, MDA, and T-SOD (Figure 2).
Cell apoptosis rate in placental tissue
The cell apoptosis rate in placental tissue in early (14.52% ± 6.82%) and late onset PE groups (16.16% ± 12.25%) was higher than the control group (5.51% ± 7.30%). However, no difference in cell apoptosis rate was seen between the PE groups (Figure 3).
Discussion
The present study showed higher SF levels in patients with PE than the normal control. Ghosh et al. 16 reported the similar findings in their study. A case-control study conducted in John Radcliffe Hospital, UK compared the serum iron status of pre-eclamptic and normal pregnant females and reported the higher iron, ferritin and transferrin saturation in pre-eclamptic females while the level of unsaturated iron and apotransferrin were significantly lower.17 Iron overload in PE condition may be the cause of disease, and the biochemical reaction can aggravate lipid peroxidation and endothelial cell damage. Increased concentrations of proteins except for ferritin in maternal serum and placental tissue during the pregnancy may suggest the wide variation in trophoblast specific proteins secretion mechanism.18
In vitro, culture of PE trophoblasts shows the increased expression and activity of reactive oxygen species (ROS) producing enzymes that may promote the transcription of antiangiogenic factors such as sFlt-1 and also inhibit the Wnt/β-catenin signaling pathway resulting in trophoblast invasion.18-20 It has been found that the expression of SOD and GSH-PX in placenta of PE patients is lower as compared with the healthy pregnant females.21 The current study reported the similar findings. The higher expression level of MDA in both PE groups, indicates the damage of placental mitochondrial membrane by high-level oxidative stress.
Regarding ferritin, about 25% is found in the form of Fe3+.22 When iron content in the body exceeds the storage capacity, it starts to deposit in reticuloendothelial system in combination with hemosiderin and excess Fe2+ generates hydroxyl radicals through Fenton reaction. It can lead to oxidative stress that may cause DNA adducts, lipid damage and cell death by initiating apoptosis.23-26 The present study showed the significantly higher levels of SF in maternal and umbilical cord blood samples in the PE groups. However, the mRNA and ferritin levels in placental samples were significantly lower compared with the controls.
Mitochondria, the main oxygen consuming organelle of the cell, shows an increased expression of ferritin. The most important function of mitochondrial ferritin (FtMt) is the isolation of excess iron in response to oxidative stress. FtMt can chelate iron ions, inhibit the production of ROS, and protects the cells from oxidative stress. In addition, intracellular ferritin consists of two different types of 24 subunits: heavy chain H-ferritin (FH) and light chain L-ferritin.27 Among them, FH has the activity to oxidize the iron that catalyzes the transformation of ferrous (Fe2+) to ferric form (Fe3+), thus allowing the iron to be incorporated into ferritin shell safely and reducing the participation of free iron in ROS production.28 The difference in protein expression seen in the current study may suggest the regulation of FTH at the transcription and post-transcription levels and thus may have a certain regulatory function on the pathological symptoms of PE.28
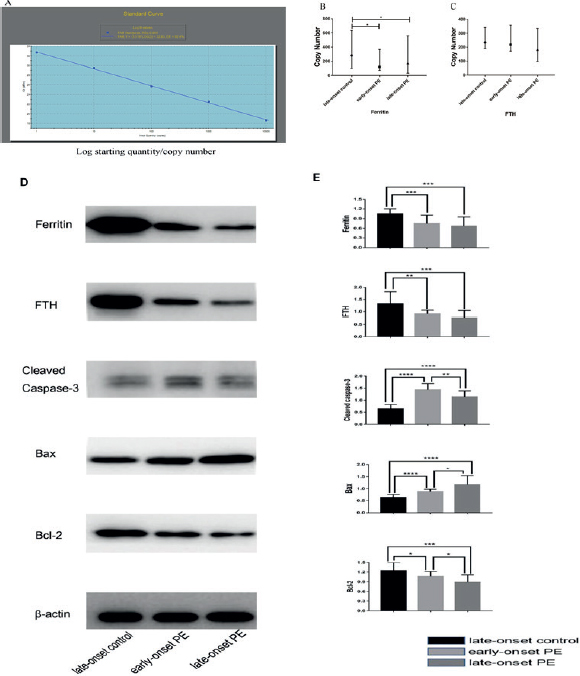
Figure 1. This figure shows the standard curve of absolute quantitative RT-qPCR (A), mRNA expression levels of ferritin and FTH (B&C), protein expression level of Ferritin, FTH, cleaved caspase-3, Bax, Bcl-2s, and beta-actin (D) and the gray value analysis result of protein bands (E).
Iron is the main post-transcriptional ferritin regulator and inhibits the iron regulatory protein (IRP), iron responsive element (IRE), and the untranslated region of ferritin H and L interaction. Messenger RNA promotes recruitment translation mechanism to produce more ferritin protein. In contrast, iron chelators stimulate IRP-IRE interaction and inhibit ferritin translation.28 Therefore, ferritin translation is up-regulated by high iron content and vice versa.
A higher degree of apoptosis has been reported in PE and placenta with restricted intrauterine growth.29 The signal pathway of apoptosis is either exogenous (death receptor) or endogenous (mitochondrial). The apoptosis of trophoblasts caused by oxidative stress is mainly achieved by endogenous pathway. The mitochondrial membrane permeability and the apoptotic proteins release are also regulated by the proteins of Bcl-2 family. There are two categories in Bcl-2 family: anti-apoptotic proteins (such as Bcl, XL) and pro apoptotic proteins (such as Bax, Bak, Mcl-2, etc.). Imbalance between the pro apoptotic (Bax, Bok, and Bak) and pro survival (Mcl-1, Bcl-2, and bcl-2l1) members of the Bcl-2 protein family and lack of oxygen supply can induce apoptosis through the endogenous pathway.30 Hypoxia has been shown to increase the Bax expression and decreases the Bcl-2 expression in human trophoblastic cells.31 Can et al.32 found the increased apoptosis index of villous trophoblasts in PE patients. The present study also confirms the difference in expression of cleaved caspase-3, Bax and other related apoptosis proteins in subjects of PE and control groups at the same time. By comparing the content of ferritin and other related proteins between the PE and the control group, this study hopes to provide some valuable reference information for the further studies regarding pathogenesis of PE with some theoretical support for the prevention of PE in clinical settings.
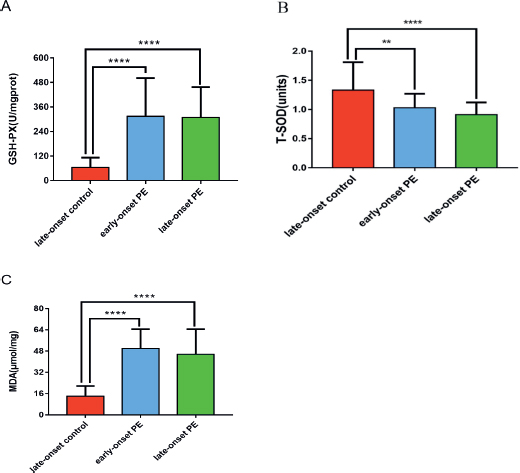
Figure 2. This figure shows the comparison of GSH-Px (A), T-SOD (B) and MDA (C) levels in placenta tissues of PE groups and late onset control groups.
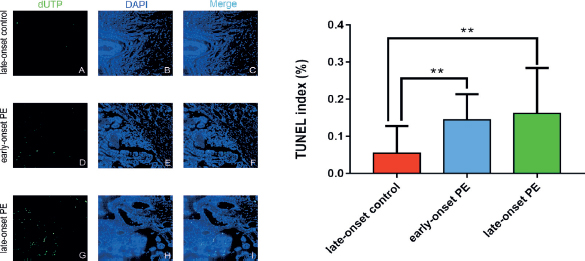
Figure 3. This figure shows DNA fragmentation (dUTP) (C, F, I) through TUNEL (A, D, G) and DAPI (B, E, H) by using double labeling with fluorescent indicator. The apoptosis rate is obtained by dividing the number of TUNEL-positive cells by the total number of DAPI-labeled nuclei (J).
Conclusion
SF levels and expression of markers of oxidative stress and cell apoptosis in blood and placental tissue of pregnant females may be considered as potential biomarkers for the diagnosis of PE. Further exploration of the link between oxidative stress, apoptosis and development of PE may be helpful in understanding the precise pathogenesis.
Limitations of the study
This study did not consider other maternal factors that may play their role in the development and progression of PE. Determination of SF levels at different stages of placental development could have been carried out.
Acknowledgement
The authors would like to thanks National Health and Family Planning Commission Science Foundation, Fujian Science and Technology and Fujian Provincial Health Technology China for their financial support and the staff at Department of Obstetrics, Fujian Provincial Maternity and Child Health Hospital, China for their co-operation.
List of Abbreviations
| BCA | Bicinchoninic acid |
| BMI | Body mass index |
| Cr | Creatinine |
| dUTP | Deoxyuridine 5-triphosphate |
| FTH | Ferritin heavy chain |
| FH | Heavy chain ferritin |
| FtMt | Mitochondrial ferritin |
| GSH-Px | Glutathione peroxidase |
| IRE | Iron responsive element |
| IRP | Iron regulatory protein |
| MDA | Malondialdehyde |
| PBS | Phosphate buffer saline |
| PE | Pre-eclampsia |
| ROS | Reactive oxygen species |
| RT-qPCR | Reverse transcription real-time fluorescence |
| SF | Serum ferritin |
| T-SOD | Superoxide dismutase |
| UA | Serum uric acid |
Conflict of interest
None to declare.
Grant support and financial disclosure
This project was financially supported by the National Health and Family Planning Commission Science Foundation (2019-WJ-04), Fujian Science and Technology Project (2018Y0005), Fujian Provincial Health Technology Project (2017-CX-11), and Key Clinical Specialty Discipline Construction of Fujian, China [(2015) no. 593].
Ethical approval
This study was approved by the institutional ethical committee of Fujian Provincial Maternity and Child Health Hospital, Fujian Medical University, China through the project No. 2017-CX-11.
Author’s contribution
JY: Conception and design of the study, analysis and interpretation of data, drafting of manuscript.
JD: Acquisition, analysis and interpretation of data, drafting of manuscript.
XL, LC, ZF, QH, QZ, LJ, XX, XL: Analysis and acquisition of data, critical revision of manuscript for important intellectual input.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ details
Jianying Yan1, Jie Dong1, Xiaoqian Lin1, Lichun Chen1, Zhuanji Fang1, Qing Han1, Qinjian Zhang1, Lingling Jiang1, Xia Xu1, Xu Lin2,3
- Department of Obstetrics, Fujian Provincial Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University, Fuzhou, China
- Key Laboratory of Ministry of Education for Gastrointestinal Cancer, School of Basic Medical Sciences, Fujian Medical University, Fuzhou, China
- Fujian Key Laboratory of Tumor Microbiology, Department of Medical Microbiology, Fujian Medical University, Fuzhou, China
References
- Croke L. Gestational hypertension and pre-eclampsia: a practice bulletin from ACOG. Am Fam Physician. 2019;100(10):649–50.
- Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of pre-eclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1–7. https://doi.org/10.1016/j.ejogrb.2013.05.005
- Mayrink J, Souza RT, Feitosa FE, Rocha Filho EA, Leite DF, Vettorazzi J, et al. Incidence and risk factors for pre-eclampsia in a cohort of healthy nulliparous pregnant females: a nested case-control study. Sci Rep. 2019;9(1):9517. https://doi.org/10.1038/s41598-019-46011-3
- Zhang C, Jin Y, Yang J. Prevalence of pregnancy induced hypertension syndrome among puerperae in Hebei province. Chin J Public Health. 2018;34(10):1395–7.
- Lee JH, Zhang G, Harvey S, Nakagawa K. Temporal trends of hospitalization, mortality, and financial impact related to pre-eclampsia with severe features in Hawai’i and the United States. Hawaii J Health Soc Welf. 2019;78(8):252–7.
- Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Pre-eclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497. https://doi.org/10.1161/CIRCOUTCOMES.116.003497
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. https://doi.org/10.1161/HYPERTENSIONAHA.117.10803
- Dai C, Zhao C, Xu M, Sui X, Sun L, Liu Y, et al. Serum lncRNAs in early pregnancy as potential biomarkers for the prediction of pregnancy-induced hypertension, including pre-eclampsia. Mol Thera Nucleic Acids. 2021;24(4):416–25. https://doi.org/10.1016/j.omtn.2021.03.010
- Zhao F, Ai F, Wu J, Dong X. Changes and clinical significance of serum inflammatory factors in the treatment of pregnancy hypertension syndrome with magnesium sulfate combined with nifedipine. Exp Ther Med. 2020;20(2):1796–802. https://doi.org/10.3892/etm.2020.8863
- Qixian T. Clinical hematology and blood test. 3th ed. Beijing, China: People’s Health Publishing House; 2003.
- Smith TA, Kirkpatrick DR, Kovilam O, Agrawal DK. Immunomodulatory role of vitamin D in the pathogenesis of pre-eclampsia. Expert Rev Clin Immunol. 2015;11(9):1055–63. https://doi.org/10.1586/1744666X.2015.1056780
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in pre-eclampsia. Obstet Gynecol. 2000;96(2):271–6. https://doi.org/10.1097/00006250-200008000-00022
- Ma HJ, Yang HL, Feng M. Expressions and significance of Survivin and caspase-3 in placenta tissue of pre-eclampsia. Matern Child Health Care of China. 2011;26(20):3148–50.
- Mendilcioglu I, Karaveli S, Erdogan G, Simsek M, Taskin O, Ozekinci M. Apoptosis and expression of Bcl-2, Bax, p53, caspase-3, and Fas, Fas ligand in placentas complicated by pre-eclampsia. Clin Exp Obstet Gynecol. 2011;38(1):38–42.
- Guidelines. The International Society for the Study of Hypertension in Pregnancy [cited 2021 Mar]. Available from: https://isshp.org/guidelines/
- Ghosh L, Afrooz R, Chowdhury SB, Gani O, Khatun R. Association between serum ferritin and iron parameters with pre-eclampsia and its association with perinatal outcome. Ibrahim Card Med J. 2019;7(1-2):64–9. https://doi.org/10.3329/icmj.v7i1-2.53961
- Rayman MP, Barlis J, Evans RW, Redman CW, King LJ. Abnormal iron parameters in the pregnancy syndrome pre-eclampsia. Am J Obstet Gynecol. 2002;187(2):412–8. https://doi.org/10.1067/mob.2002.123895
- Lim R, Acharya R, Delpachitra P, Hobson S, Sobey CG, Drummond GR, et al. Activin and NADPH-oxidase in pre-eclampsia: insights from in vitro and murine studies. Am J Obstet Gynecol. 2015;212(1):86e1–12. https://doi.org/10.1016/j.ajog.2014.07.021
- Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in pre-eclampsia. Am J Pathol. 2000;156(1):321–31. https://doi.org/10.1016/S0002-9440(10)64733-5
- Huang QT, Wang SS, Zhang M, Huang LP, Tian JW, Yu YH, et al. Advanced oxidation protein products enhances soluble Fms-like tyrosine kinase 1 expression in trophoblasts: a possible link between oxidative stress and pre-eclampsia. Placenta. 2013;34(10):949–52. https://doi.org/10.1016/j.placenta.2013.06.308
- Zhuang B, Luo X, Rao H, Li Q, Shan N, Liu X, et al. Oxidative stress-induced C/EBPβ inhibits β-catenin signaling molecule involving in the pathology of pre-eclampsia. Placenta. 2015;36(8):839–46. https://doi.org/10.1016/j.placenta.2015.06.016
- Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of pre-eclampsia. Hypertens Pregnancy. 2002;21(3):205–23. https://doi.org/10.1081/PRG-120015848
- Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of iron homeostasis: new players in metabolism, cell death, and disease. Trends Biochem Sci. 2016;41(3):274–86. https://doi.org/10.1016/j.tibs.2015.11.012
- Crooks DR, Maio N, Lane AN, Jarnik M, Higashi RM, Haller RG, et al. Acute loss of iron-sulfur clusters results in metabolic reprogramming and generation of lipid droplets in mammalian cells. J Biol Chem. 2018;293(21):8297–311. https://doi.org/10.1074/jbc.RA118.001885
- Chen Y, Li J, Wei J, Kawan A, Wang L, Zhang X. Vitamin C modulates microcystis aeruginosa death and toxin release by induced Fenton reaction. J Hazard Mater. 2017;321:888–95. https://doi.org/10.1016/j.jhazmat.2016.10.010
- Rayman MP, Barlis J, Evans RW, Redman CW, King LJ. Abnormal iron parameters in the pregnancy syndrome pre-eclampsia. Am J Obstet Gynecol. 2002;187(2):412–8. https://doi.org/10.1067/mob.2002.123895
- Quan YY, Qin GQ, Huang H, Liu YH, Wang XP, Chen TS. Dominant roles of Fenton reaction in sodium nitroprusside-induced chondrocyte apoptosis. Free Radic Biol Med. 2016;94(4):135–44. https://doi.org/10.1016/j.freeradbiomed.2016.02.026
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochem Biophys Acta. 1996;1275(3):161–203. https://doi.org/10.1016/0005-2728(96)00022-9
- Huang BW, Miyazawa M, Tsuji Y. Distinct regulatory mechanisms of the human ferritin gene by hypoxia and hypoxia mimetic cobalt chloride at the transcriptional and post-transcriptional levels. Cell Signal. 2014;26(12):2702–9. https://doi.org/10.1016/j.cellsig.2014.08.018
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either pre-eclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186(1):158–66. https://doi.org/10.1067/mob.2002.119176
- Shroff EH, Snyder C, Chandel NS. Bcl-2 family members regulate anoxia-induced cell death. Antioxid Redox Signal. 2007;9(9):1405–9. https://doi.org/10.1089/ars.2007.1731
- Can M, Guven B, Bektas S, Arikan I. Oxidative stress and apoptosis in pre-eclampsia. Tissue Cell. 2014;46(6):477–81. https://doi.org/10.1016/j.tice.2014.08.004
