Original Article
Volume: 37 | Issue: 4 | Published: Dec 25, 2021 | Pages: 227 - 233 | DOI: 10.51441/BioMedica/5-557
Anatomical Parameters of Eye and Associated Histological Features are Potential Risk Factors for Development of Pterygium
Authors: Sadia Aman , Muhammad Muneeb Ather , Amanullah Qasim , Rabia Latif , Rakhshanda Jabeen , Uruj Zehra
Article Info
Authors
Sadia Aman
Department of Anatomy, University of Health Sciences, Lahore, Pakistan
Muhammad Muneeb Ather
Department of Anatomy, University of Health Sciences, Lahore, Pakistan
Amanullah Qasim
Department of Ophthalmology, Allied Hospital Faisalabad, Pakistan
Rabia Latif
Department of Anatomy, University of Health Sciences, Lahore
Rakhshanda Jabeen
Department of Anatomy, Sahara Medical College, Narowal, Pakistan
Uruj Zehra
Department of Anatomy, University of Health Sciences, Lahore, Pakistan
Publication History
Received: November 18, 2021
Accepted: December 14, 2021
Published: December 25, 2021
Abstract
Background and Objectives: Pterygium occurs throughout the world but the exact pathogenesis is still not clear. The reports on the association between eye anatomical parameters and presence of pterygium is controversial, similarly how the histological features of the pterygium may differ due to these parameters is not known. Thus, the focus of this study was to explore this gap by assessing and comparing the anatomical parameters of eye in pterygium patients and in healthy controls. In addition, histological features of pterygium were assessed and correlated with the eye anatomical parameters of the patients.
Methods: Forty-one pterygium patients of age range 25-70 years undergoing surgery were included in the study after taking written informed consent. Forty-two age and sex matched healthy controls were also recruited for assessing anatomical eye parameters. Relevant history with demographic details of every subject was obtained. All participants underwent a thorough ophthalmic examination. Assessment of orbital protrusion and interpalpebral distance was measured by millimeter scale. Tear film breakup time (TFBUT) and Schirmer test was used for tear film assessment. Presence of any meibomian gland dysfunction (MGD) was examined by slit lamp. Post-surgical tissue samples from patients were assessed for histological features with H& E, PAS & Verhoeff stains. Data were processed and analyzed by using SPSS version 21.0.
Results: Eyeball protrusion and MGD was found higher in pterygium patients as compared to controls. Basement membrane fragmentation on histology significantly correlated with the eyeball protrusion (P<0.04) and TFBUT (P<0.020), inflammation significantly correlated with the MGD (P<0.05) while elastosis showed significant correlation with TFBUT (P<0.001).
Conclusion: It can be concluded that eye anatomical parameters might be the risk factors in the development of pterygium. The correlation between certain histological features & eye anatomical parameters indicate that anatomical eye parameters can be taken as risk factor for recurrence in these patients.
Keywords: Pterygium, Meibomian Gland Dysfunction, Eyeball Protrusion, Interpalpebral distance
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(4):227-233
ORIGINAL ARTICLE
Anatomical parameters of eye and associated histological features are potential risk factors for development of pterygium
Sadia Aman1, Muhammad Muneeb Ather1, Amanullah Qasim2, Rabia Latif1, Rakhshanda Jabeen3, Uruj Zehra1*
Received: 18,November,2021 Revised date: 02 December,2021 Accepted: 14 December,2021
Correspondence to: Uruj Zehra
*Department of Anatomy, University of Health Sciences, Lahore, Pakistan.
Email: uruj.zehra@gmail.com
Full list of author information is available at the end of the article.
ABSTRACT
Background and Objective:
Pterygium occurs throughout the world, but the exact pathogenesis is still not clear. The reports on the association between eye anatomical parameters and presence of pterygium is controversial, similarly how the histological features of the pterygium may differ due to these parameters is not known. Thus, the focus of this study was to explore this gap by assessing and comparing the anatomical parameters of eye in pterygium patients and in healthy controls. In addition, histological features of pterygium were assessed and correlated with the eye anatomical parameters of the patients.
Methods:
Forty-one pterygium patients of age range 25-70 years undergoing surgery were included in the study after taking written informed consent. Forty-two age and sex matched healthy controls were also recruited for assessing anatomical eye parameters. Relevant history with demographic details of every subject was obtained. All participants underwent a thorough ophthalmic examination. The assessment of orbital protrusion and interpalpebral distance was measured by millimeter scale. Tear film breakup time (TFBUT) and Schirmer test were used for tear film assessment. Presence of any meibomian gland dysfunction (MGD) was examined by slit lamp. Postsurgical tissue samples from patients were assessed for histological features with Haematoxylin and Eosin, Periodic acid Schiff & Verhoeff stains. Data were processed and analyzed by using SPSS version 23.0.
Results:
Eyeball protrusion and MGD were found higher in pterygium patients as compared to controls. Basement membrane fragmentation on histology significantly correlated with the eyeball protrusion (p < 0.04) and TFBUT (p < 0.020), inflammation significantly correlated with the MGD (p < 0.05), while elastosis showed significant correlation with TFBUT (p < 0.001).
Conclusion:
It can be concluded that eye anatomical parameters might be the risk factors in the development of pterygium. The correlation between certain histological features and eye anatomical parameters indicate that anatomical eye parameters can be taken as risk factor for recurrence in these patients.
Keywords:
Pterygium, anatomy, meibomian gland dysfunction, eyeball protrusion, interpalpebral distance, histology.
Introduction
Pterygium is a triangular fibro vascular degenerative bulbar connective tissue growth that encroaches the cornea1 and is more common in people who live at equator, with history of increased sun exposure.2 According to a local study done in Pakistan on 1,227 pterygium patients, the prevalence was found slightly higher in individuals of hot and dry weather areas.3 The existing literature mostly reports this condition on clinical grounds that is regarding its management and recurrence, but lesser and debatable data is available concerning its etiology, pathogenesis, and histological features which can trigger recurrence. The specific cause of pterygium is still unknown,1 the studies which were done on different aspects of pterygium create curiosity regarding the pathogenesis and also provide better clues to hypothesize the relationship of possible factors to pterygium presence and recurrence.
Literature emphasized that ultraviolet (UV) rays of sun, warm, and dusty environment play significant role in pterygium development.4 Regarding the risk factors, few studies report the association between eye parameters, such as protrusion, interpalpebral distance, and the occurrence of pterygium suggesting that anatomical parameters of eye may affect the development of pterygium by making eyes more prone to UV exposure.1,5,6 Dry eyes in association with decreased tear film break up time (TFBUT) and meibomian gland dysfunction (MGD) is also reported to be among risk factors for pterygium.7,8,9,10,11
The management of pterygium is usually the surgical excision, but chances of the recurrence are quite substantial. Limited data suggests that the features of pterygium vary from person to person and can be a predictive factor for recurrence. Convincing and detailed studies, however, are required to explore the histological features of pterygium, their association with any other eye parameters.
The current study was designed to assess the eye anatomical parameters in patients with pterygium and compare with the same parameters of the healthy controls. The eye anatomical parameters were also associated with the histological features of pterygium. This study will help the ophthalmologists to identify potential patients of pterygium development and risk, and associated visual impairment can be minimized. The association with histological features will help understand the pathogenesis of the condition and suitable treatment options.
Methods
Forty-one patients with pterygium and 42 healthy controls were recruited after taking ethical approval from the institutional ethical review committee. Informed written consent was taken to examine the participants’ eyes and for collection of samples of pterygium after surgery for the purpose of research. Patients with unilateral pterygium from 25 to 70 years undergoing pterygium excision were included from the Department of Ophthalmology, Allied Hospital Faisalabad, Pakistan. Histology and special staining was performed at the Department of Anatomy, University of Health Sciences, Lahore Pakistan from February 2019 to December 2019. Age and sex matched individuals with no past, personal, and family history of pterygium were taken as controls for eye anatomical parameters observation. Patients who were on medical treatment for pterygium including topical steroids or non-steroidal anti-inflammatory drugs, had previous ocular surgery, conjunctival cicatricle disease, systemic autoimmune disease, untreated dry eye disease, contact lens users, patients with anterior or posterior segment disease which alters tear secretion, patients on topical and glaucoma medications that leads to ocular drying, those with pseudo-pterygium were excluded from the study.
The complete demographic and clinical history was taken from both patients and controls, and anatomical parameters of eyes were checked by an experienced ophthalmologist. Patients were examined for inter palpebral distance, eyeball protrusion, tear film time through Schirmer test, TFBUT and meibomian gland (MG) morphology (posterior shifting of muco cutaneous margin, plugging of MG orifice and MG dropout) before performing surgery. Pterygium samples were received after surgery in 10% formalin and were fixed for 48-72 hours.
The fixed tissues were processed and paraffin embedded sections were stained with Hematoxylin and Eosin (H&E), Periodic acid Schiff (PAS), and Verhoeff stain. Following histological parameters were studied and graded in pterygium tissue.12,13
Basement membrane fragmentation
Uniformity of the basement membrane was observed on PAS stain, any discontinuity, and fragmentation was recorded as grade1, while absence was recorded as grade 0.12,13
Goblet cells in surface epithelium
Goblet cells were also observed on PAS stain. If goblet cells were in the form of single cells, they were considered grade 0. Goblet clusters were taken as grade 1, and in case of invagination of goblets cells into underlying epithelium, they were taken as grade 2.12,13
Stromal infiltration of inflammatory cells
Different leukocytes including neutrophils, monocyte, macrophages, and lymphocytes were observed under 40× objective lens. They were observed on the basis of either few or diffusely scattered cells. Four different fields were chosen in a slide, if there were diffusely scattered cells in more than two fields then it was considered as grade-1. Otherwise, they were taken as grade 0 in case of patchy distribution. These observations were taken with the help of H&E and PAS stain.12,13
Elastosis
Elastosis was observed on Verhoeff stain as characteristic wavy fibers, in case of no elastosis, grade 0 was given. If elastosis is less than 3% of the field, then it was grade 1, but if it was 3%-10% it was grade 2 and if >10% it was recorded as grade 3.12,13
Statistical analysis
Collected data was processed and analyzed by using SPSS software version 23. Mean ± SD was calculated for quantitative variables (eyeball protrusion, IP distance, and tear film). Frequency and percentages were calculated for categorical data (i.e., MG and histological parameters).
Independent sample t-test was applied to compare the quantitative parameters (eyeball protrusion, IP distance, tear film, MG) among the groups. Chi-square test was applied to see relation between the histological parameters with eyeball protrusion, IP distance, Schirmer test, and TFBUT. A p value ≤ 0.05 was considered to indicate the significance.
Results
Among the pterygium patients there were 58.5% females and 41.5% males, while in controls, 50% were males, no significant difference was observed. In pterygium group, males were having significantly greater age compared to females (p = 0.01). The demographic history revealed that pterygium cases were more exposed (p < 0.001) to sunlight (4 to 6 hours/day) as compared to controls (2 to 3 hours/ day). A total of 36% smokers were found within pterygium group as compared to controls which was 12% (p = 0.009). Toxic chemical exposure time (7 to 8 hours/day) was highly significant in pterygium patients as compared to controls (0 to 2 hours) (p < 0.001)
Eyeball protrusion was significantly higher (p < 0.001) in pterygium patients (mean ± SD; 15.00 ± 2.9) as compared to controls (mean ± SD; 12.12 ± 1.9). There was no significant difference of IP distance, Schirmer test and TFBUT results between pterygium patients and controls (Table 1, Figure 1)
MGD was significantly higher (p = 0.008) in pterygium patients (53.7%) as compared to controls (23.8%). When different morphological features of MG (posterior shifting, plugging, drop out) were compared between patients and controls, gland drop out was found significant (p = 0.002) in pterygium patients (Table 1).
On histological examination of pterygium, distribution of goblet cells within the stratified squamous epithelium was observed. Single goblet cells (grade 0) were seen in 22% of the sections, 17.1% sections showed cluster of cells (grade 1), and 61.1% showed invagination of goblet cells (grade 2) into underlying connective tissue (Figure 2).
Basement membrane fragmentation was found in 95.1% of tissues (Figure 2). Below the basement membrane, the connective tissue stroma contained inflammatory cells, elastotic fibers, fibroblasts, and stromal fibers scattered throughout the connective tissue. Grade 1 infiltration of inflammatory cells was found in 90.2% of the samples (Figure 2).
Table 1. Comparison of MGD and morphological features of Meibomian gland between patients (cases) and controls.
| Groups | MGD | Posterior shifting | Plugging | Drop out |
|---|---|---|---|---|
| Cases | 53.7% (22) ** | 4.9% | 29.3%* | 22%** |
| Controls | 23.8% (10) | 11.9% | 16.7% | 0 |
* p < 0.01, **p < 0.001.
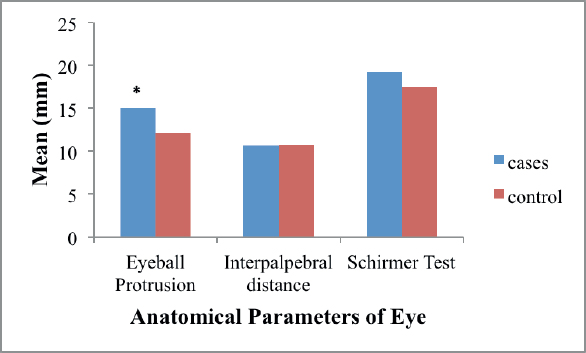
Figure 1. Graph showing comparison of anatomical parameters of eye between pterygium patients (cases) and controls. * p < 0.001.
Elastotic fibers were seen spiral in morphology which can be easily differentiated from other fibers by the Verhoeff stained sections. Grade 0 was not found in any section, elastosis with grade 1 was found in only one sample, almost 19.8% samples showed grade 2 elastosis, and 78% with grade 3 elastosis were seen (Figure 3).
Among the anatomical eye parameters, basement membrane fragmentation was found significantly related to the eyeball protrusion (p = 0.04) and TFBUT (p = 0.02), diffuse infiltration of inflammatory cells was in borderline significant association with the MGD (p = 0.05), while higher grades of elastosis were seen in patients with decreased TFBUT (p < 0.001) (Table 2).
Discussion
Current study revealed significant difference of eyeball protrusion and MGD between pterygium patients and controls. On histology, the basement membrane fragmentation was in significant association with the eyeball protrusion and TFBUT. Inflammation was in a significant association with MGD and increased elastosis was seen in patients with decreased TFBUT.
Increased eyeball protrusion in pterygium patients as compared to control is in agreement with the findings of previous report.14 The protruded eyeball is more prone to be exposed to UV rays leading to pterygium formation; the significantly higher eyeball protrusion in the pterygium group in the current study can be explained by this.
The current study showed higher MGD in pterygium patients in comparison to the controls, which is similar to the previous results.15-18 This can be explained on the basis of the notion that the direct contact of pterygium with palpebral conjunctiva squeezes the MGs which leads to alterations in glands. Still, a detailed study is needed to determine the pathological process of MGD and unstable tear film in pterygium patients. The various morphological features of MGD, plugging and dropout of MGs which were found statistically higher in pterygium patients in the current study were also found in the previous study.19 The possible hypothesis of these findings might be related to the chronic inflammatory process in the pterygium tissue. The process of inflammations of MGs in pterygium patients blocks the meibum secretion and causes orifice keratinization.20 This can be possible etiology in the current study, as the MGD was also significantly correlated with infiltration of inflammatory cells.
Basement membrane fragmentation was in a significant association with the eyeball protrusion and TFBUT. This can be explained by a presumption that increased eyeball protrusion is associated with high UV exposure leading to tear evaporation. Dry eye may be the reason behind the ocular surface damage resulting in the basement membrane fragmentation.
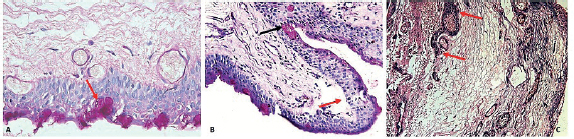
Figure 2 Histological images of pterygium tissue showing different features A) cluster of goblet cells (red arrow) PAS stained ×40 (B) Basement membrane fragmentation beneath epithelium (red arrow) and invagination of goblet cells (black arrow) PAS stained ×40 (C) diffuse infiltration of inflammatory cells (red arrows H&E stained ×10).
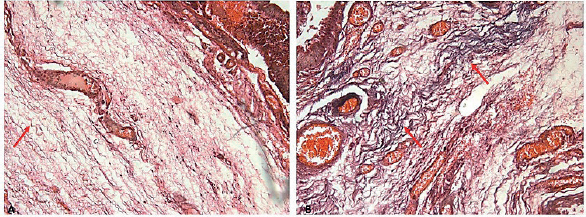
Figure 3. Verhoeff stained sections showing elastosis of different grades (red arrows) Note congested blood vessels in the tissue sections ×10.
Table 2. Correlation of anatomical parameters of eye with histological parameters.
| Histological changes | Basement membrane fragmentation | Goblet cells | Leukocyte infiltration | Elastosis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grades | 0 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 2 | 3 |
| Eyeball protrusion (mean ± SD) | 11 ± 1.4 | 15.21 ± 2.8* | 14.6 ± 2.7 | 16 ± 3.0 | 14.9 ± 3.01 | 16 ± 2 | 14 ± 2 | 11 | 15 ± 2 | 15 ± 2 |
| IP distance (mean ± SD) | 11 ± 1.4 | 10.6 ± 1.53 | 11 ± 1.2 | 11 ± 1.1 | 10.7 ± 1.7 | 10.6 ± 1 | 10.5 ± 2.6 | 13 | 11 ± 1 | 10 ± 1.5 |
| TFBUT (mean ± SD) | 9 ± 4.2* | 5.1 ± 2 | 6.11 ± 3 | 4.6 ± 1.1 | 5.3 ± 2.2 | 6 ± 2.9 | 5.3 ± 2 | 7 | 8 ± 2.6 | 4.6 ± 1.6** |
| Schirmer test (mean ± SD) | 21 ± 12.7 | 21 ± 1,219 ± 9.4 | 20 ± 11 | 19.3 ± 8 | 19 ± 9.1 | 24 ± 12 | 18 ± 9 | 13 | 18 ± 9.28 | 19.6 ± 9.6 |
| MGD (%) | 4.5% | 95.4% | 22.7% | 13.6% | 63.6% | 18.2% | 81.8%* | 4.5% | 22.7% | 72.7% |
IP: Interpalpebral distance, TFBUT: Tear film breakup time, MGD: Meibomian gland dysfunction.
*p < 0.05, **p < 0.001.
Stromal infiltration of inflammatory cells was highly associated with MGD. Due to the cross-sectional design of the study, it is hard to understand the cause and effect relationship and it seems difficult to suggest that whether inflammation of the pterygium caused the MGD or vice versa but this correlation can be explained with the possibility that inflammation of the pterygium results in the release of inflammatory cytokines causing alterations in MGs. Moreover, it is also hypothesized that meibomitis induced by any infection produces unstable lipid layer of the tear film which disturbs the normal organization of cornea, and thus can cause inflammation.21-23
The elastosis was seen significantly associated with TFBUT. The pathogenesis may be associated with solar elastosis, as it leads to disturbed elastin deposition. Sun rays produce increased activity of matrix metalloproteinase and human macrophage elastase which leads to fibrohexis and fibrolysis24,25 disturbing the tear film layer.
To the best of authors’ knowledge, this study is the first to associate the anatomical parameters of eye and histological features of pterygium. The studyfor the first time recorded and observed the differences in the eye anatomical parameters and gross features of pterygium in the local population. The correlation between eye anatomical parameters and pterygium in the local population were observed meticulously and pattern was recorded. Interestingly, two out of three eye anatomical parameters which were different between cases and controls were also in correlation with the histological features. This finding strengthens the reliability of our results and advocates that eye ball protrusion and MGD might be the two important factors, ophthalmologists need to focus on while treating the patient of pterygium.
Conclusion
The difference in anatomical parameters of eye (eyeball protrusion and MGD) between pterygium patients and controls and their strong correlation with the histological features suggests that anatomical variations might be the risk factors in the occurrence and recurrence of the pterygium.
Limitations of the study
Like every other study, this study also has certain limitations. First: it is the cross-sectional study, and the authors could only show the simple association between parameters, and cause and effect relationship could not be ascertained. The reason being the restricted time duration for the academic research and limited resources, but through this study we were able to understand the possible link and can recommend the prospective study in future.
Second: the sample population, due to limited time and resources constraint, we could only include patients from Faisalabad. The inclusion of the patients from different areas of Pakistan will give a better picture and data of these parameters.
Acknowledgement
The authors would like to acknowledge Ms. Bushra Munir for helping in special staining and photomicrography of the sample tissues. The authors would also like to thank the staff of the Department of Ophthalmology, Allied Hospital Faisalabad, Pakistan for their logistic support during sampling.
List of Abbreviations
| H&E | Haematoxylin and Eosin |
| MG | Meibomian gland |
| MGD | Meibomian gland dysfunction |
| PAS | Periodic acid Schiff |
| TFBUT | Tear film breakup time |
| UV | Ultraviolet |
Conflict of interest
None to declare.
Grant support and financial disclosure
This research work was supported by University of Health Sciences, Lahore Pakistan.
Ethical approval
The study was approved by the Ethical Review Committee of University of Health Sciences, Lahore, Pakistan via reference letter number UHS/REG-19/ERC/2484 dated July 17, 2019.
Authors’ Contribution
SA: Acquisition, analysis and interpretation of data, drafting of manuscript.
MMA, RL: Analysis and interpretation of data, drafting of the manuscript.
AQ: Data acquisition and analysis.
RJ: Interpretation and analysis of data.
UZ: Concept and design of study, interpretation of data, critical revision of manuscript for important intellectual content.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ details
Sadia Aman1, Muhammad Muneeb Ather1, Amanullah Qasim2, Rabia Latif1, Rakhshanda Jabeen3, Uruj Zehra1
- Department of Anatomy, University of Health Sciences, Lahore, Pakistan
- Department of Ophthalmology, Allied Hospital Faisalabad, Pakistan
- Department of Anatomy, Sahara Medical College, Narowal, Pakistan
References
- Altinkaynak H, Demircan A, Kocasarac C, Kara N, Dundar H, Altan Ç, et al. Effect of orbital protrusion and vertical interpalpebral distance on pterygium formation. Cont Lens Anterior Eye. 2014;37(3):153–6. https://doi.org/10.1016/j.clae.2013.09.010
- McGlacken-Byrne AB, Drinkwater JJ, Mackey DA, Turner AW. Gender and ethnic differences in pterygium prevalence: an audit of remote Australian clinics. Clin Exp Ophthalmol. 2021;104 (1):74–7. https://doi.org/10.1111/cxo.13081
- Shah SIA, Shah SA, Rai P. Factors associated with pterygium based on history and clinical examination of patients in Pakistan. J Curr Ophthalmol. 2016;28(2):91–2. https://doi.org/10.1016/j.joco.2016.03.005
- Humayun J, Farhan M, Kamran MK, Khan SAR. Recurrence rate of primary pterygium following excision with mitomycin c versus excision with amniotic membrane transplant. Pak J Ophthalmol. 2020;36(3):1–5. https://doi.org/10.36351/pjo.v36i3.1033
- Saw SM, Banerjee K, Tan D. Risk factors for the development of pterygium in Singapore: a hospital-based case-control study. Acta Ophthalmol Scand. 2000;78(2):216–20. https://doi.org/10.1034/j.1600-0420.2000.078002216.x
- Ozer PA, Altiparmak UE, Yalniz Z, Kasim R, Duman S. Prevalence of pinguecula and pterygium in patients with thyroid orbitopathy. Cornea. 2010;29(6):659–63. https://doi.org/10.1097/ICO.0b013e3181c296ab
- Kampitak K, Leelawongtawun W. Precorneal tear film in pterygium eye. J Med Assoc Thai. 2014;97(5):536–9. PMID: 25065094
- Balogun M, Ashaye A, Ajayi B, Osuntokun O. Tear break-up time in eyes with pterygia and pingueculae in Ibadan. West Afr J Med. 2005;24(2):162–6. https://doi.org/10.4314/wajm.v24i2.28189
- Pflugfelder SC, de Paiva CS. The pathophysiology of dry eye disease: what we know and future directions for research. Ophthalmology. 2017;124(11):S4–13. https://doi.org/10.1016/j.ophtha.2017.07.010
- Ishioka M, Shimmura S, Yagi Y, Tsubota K. Ptyerygium and dry eye. Ophthalmologica. 2001;215(3):209–11. https://doi.org/10.1159/000050860
- Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol (Auckland, NZ). 2016;10:1385. https://doi.org/10.2147/OPTH.S109663
- Chui J, Coroneo MT, Tat LT, Crouch R, Wakefield D, Di Girolamo N. Ophthalmic pterygium: a stem cell disorder with premalignant features. Am J Pathol. 2011;178(2):817–27. https://doi.org/10.1016/j.ajpath.2010.10.037
- Hashemian H, Mahbod M, Amoli FA, Kiarudi MY, Jabbarvand M, Kheirkhah A. Histopathology of conjunctivochalasis compared to normal conjunctiva. J Ophthalmic Vis Res. 2016;11(4):345. https://doi.org/10.4103/2008-322X.194068
- Demirok A, Cinal A, Yener HI, Yasar T, Kilic A. The risk factors of pterygium development: a hospital-based study. Ann Ophthalmol (Skokie). 2008;40(2):103–6. PMID: 19013918
- Wu H, Lin Z, Yang F, Fang X, Dong N, Luo S, et al. Meibomian gland dysfunction correlates to the tear film instability and ocular discomfort in patients with pterygium. Sci Rep. 2017;7(1):1–8. https://doi.org/10.1038/srep45115
- Wanzeler ACV, Barbosa IAF, Duarte B, Barbosa EB, Borges DA, Alves M. Impact of pterygium on the ocular surface and meibomian glands. PloS One. 2019;14(9):e0213956. https://doi.org/10.1371/journal.pone.0213956
- Li N, Wang T, Wang R, Duan X. Tear film instability and meibomian gland dysfunction correlate with the pterygium size and thickness pre-and postexcision in patients with pterygium. J Ophthalmol. 2019;2019:1–9. https://doi.org/10.1155/2019/5935239
- Das P, Gokani A, Bagchi K, Bhaduri G, Chaudhuri S, Law S. Limbal epithelial stem-microenvironmental alteration leads to pterygium development. Mol Cell Biochem. 2015;402(1- 2):123–39. https://doi.org/10.1007/s11010-014-2320-z
- Ye F, Zhou F, Xia Y, Zhu X, Wu Y, Huang Z. Evaluation of meibomian gland and tear film changes in patients with pterygium. Indian J Ophthalmol. 2017;65(3):233. https://doi.org/10.4103/ijo.IJO_743_16
- Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Shimazaki J, et al. In vivo confocal microscopy evaluation of meibomian gland dysfunction in atopic-keratoconjunctivitis patients. Ophthalmology. 2012;119(10):1961–8. https://doi.org/10.1016/j.ophtha.2012.04.001
- Suzuki T, Kinoshita S. Meibomitis-related keratoconjunctivitis in childhood and adolescence. Am J Ophthalmol. 2007;144(1):160–1.
- Suzuki T. Meibomitis-related keratoconjunctivitis: implications and clinical significance of meibomian gland inflammation. Cornea. 2012;31:S41–4. https://doi.org/10.1016/j.ajo.2007.04.017
- Suzuki T. Inflamed obstructive meibomian gland dysfunction causes ocular surface inflammation. Invest Ophthalmol Vis Sci. 2018;59(14):DES94–101. https://doi.org/10.1167/iovs.17-23345
- Chung JH, Seo JY, Lee MK, Eun HC, Lee JH, Kang S, et al. Ultraviolet modulation of human macrophage metalloelastase in human skin in vivo. J Invest Dermatol. 2002;119(2):507–12. https://doi.org/10.1046/j.1523-1747.2002.01844.x
- Dhital B, Durlik P, Rathod P, Gul-E-Noor F, Wang Z, Sun C, et al. Ultraviolet radiation reduces desmosine cross-links in elastin. Biochem Biophys Rep. 2017;10(1):172–7. https://doi.org/10.1016/j.bbrep.2017.04.002
