Review Article
VOLUME: 37 | ISSUE: 3 | Sep 25, 2021 | PAGE: (139 - 147) | DOI: 10.51441/BioMedica/5-498
The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges
Authors: Cui Lianxu , Yasmeen Saeed , Li Haomin , Yang Jingli
Article Info
Authors
Cui Lianxu
Department of Neurosurgery, The First People’s Hospital of Foshan, No.81 North Lingnan Road, Foshan, Guangdong - PR China.
Yasmeen Saeed
Guangdong VitaLife Biotechnology Co., LTD, No. 61 Xiannan Road, Nanhai District, Foshan, Guangdong - PR China.
Li Haomin
Department of Neurosurgery, The First People’s Hospital of Foshan, No.81 North Lingnan Road, Foshan, Guangdong - PR China.
Yang Jingli
School of Medicine, Foshan University, 18 Jiangwan Road, Foshan, Guangdong -PR China.
Publication History
Received: June 18, 2021
Revised: July 29, 2021
Accepted: September 11, 2021
Published: September 25, 2021
Abstract
Traumatic brain injury (TBI) is a focal injury with limited reliable treatment options. Despite the large volume of basic research into TBI (particularly on the complex pathophysiology and on the application of various techniques), the treatment of TBI currently remains a challenge due to the low efficacy of available therapeutic options. Recent studies have shown that stem cells possess the ability to aid in recovery from the damaging effects of the craniocerebral injury. Herein, we attempted to present a generalized critique for the role of mesenchymal stem cell therapy in TBI, its underlying mechanisms, and the scope for improvements in TBI treatment identified through preclinical studies, clinical studies, and other research in the light of previously reported literature. Finally, we summarized some novel strategies to overcome the clinical challenges in TBI recovery. Collectively, the major objective of this review is to highlight the to-date available findings regarding role of stem cell therapy in TBI and pave the way for the development of safe and efficient regenerative treatment modalities for TBI by comprehensive understanding the specific mechanism.
Keywords: Traumatic brain injury, Mesenchymal stem cells, Stem cell therapy, Molecular mechanism, Regenerative medicine.
Pubmed Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli. The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges. BioMedica. 2021; 25 (September 2021): 139-147. doi:10.51441/BioMedica/5-498
Web Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli. The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges. https://biomedicapk.com/articles/online_first/498 [Access: July 03, 2024]. doi:10.51441/BioMedica/5-498
AMA (American Medical Association) Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli. The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges. BioMedica. 2021; 25 (September 2021): 139-147. doi:10.51441/BioMedica/5-498
Vancouver/ICMJE Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli. The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges. BioMedica. (2021), [cited July 03, 2024]; 25 (September 2021): 139-147. doi:10.51441/BioMedica/5-498
Harvard Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli (2021) The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges. BioMedica, 25 (September 2021): 139-147. doi:10.51441/BioMedica/5-498
Chicago Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli. "The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges." 25 (2021), 139-147. doi:10.51441/BioMedica/5-498
MLA (The Modern Language Association) Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli. "The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges." 25.September 2021 (2021), 139-147. Print. doi:10.51441/BioMedica/5-498
APA (American Psychological Association) Style
Cui Lianxu, Yasmeen Saeed, Li Haomin, Yang Jingli (2021) The Role of Mesenchymal Stem Cell Therapy in Traumatic Brain Injury: A Review from Basic Research to Clinical Challenges. , 25 (September 2021), 139-147. doi:10.51441/BioMedica/5-498
Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(3):139-147
REVIEW ARTICLE
The role of mesenchymal stem cell therapy in traumatic brain injury: a review from basic research to clinical challenges
Cui Lianxu1, Yasmeen Saeed2, Li Haomin1, Yang Jingli3*
Received: 18 June 2021 Revised date: 29 July 2021 Accepted: 11 September 2021
Correspondence to: Yang Jingli
*School of Medicine, Foshan University, Foshan, China.
Email: yangjingli@fosu.edu.cn
Full list of author information is available at the end of the article.
ABSTRACT
Traumatic brain injury (TBI) is a focal injury with limited reliable treatment options. Despite the large volume of basic research into TBI (particularly on the complex pathophysiology and on the application of various techniques), the treatment of TBI currently remains a challenge due to the low efficacy of available therapeutic options. Recent studies have shown that stem cells possess the ability to aid in recovery from the damaging effects of the craniocerebral injury. Herein, we attempted to present a generalized critique for the role of mesenchymal stem cell therapy in TBI, its underlying mechanisms, and the scope for improvements in TBI treatment identified through preclinical studies, clinical studies, and other research in the light of previously reported literature. Finally, we summarized some novel strategies to overcome the clinical challenges in TBI recovery. Collectively, the major objective of this review is to highlight the to-date available findings regarding role of stem cell therapy in TBI and pave the way for the development of safe and efficient regenerative treatment modalities for TBI by comprehensive understanding the specific mechanism.
Keywords:
Traumatic brain injury, mesenchymal stem cells, stem cell therapy, molecular mechanism, regenerative medicine.
Introduction
Traumatic brain injury (TBI) is caused by an external mechanical force1 causing either temporary damage or permanent dysfunctions and ultimately results in severe cognitive, physical, and emotional disorders.2,3 The recent decade has seen a dire increase in the rate of TBI4 and which make it the third leading cause of death worldwide.5,6 However, despite being a major cause of death and disability throughout the world7,8, to date, available treatments are only designed to combat the symptoms of primary injury to prevent the further progression of damage.8
TBI is categorized on the basis of clinical severity and is primarily assessed using the Glasgow Coma Scale (GCS).9,10 According to this classification, a GCS score of 13-15 is assigned to mild TBI, while 9-12 of GCS score has been attributed to the moderate TBI, and a GCS notch of ≤8 indicates the severe TBI, respectively.10,11 Besides GCS, various imaging modalities are used to evaluate the severity of structural damage in the brain caused by TBI.
Although, for patients suffering from serious injuries, surgical intervention is critically required for the detection and management of high intracranial pressure.12 Whereas, brain stimulation, hyperbaric oxygen, and behavioral therapy are commonly applied clinical strategies to treat neurological dysfunction after TBI.13 However, to date, these strategies have not exhibited any satisfactory remedial efficiency. Meanwhile, the heterogeneity of patients and injury along with the wide range of its clinical manifestations remained the significant challenge for the development of effective treatment and diagnostic strategies for TBI.14 Therefore, the identification of reliable prognostic markers, an efficiently modified detection method and effective yet safe treatment are required for the management of acute and chronic injuries.9
Though accrued studies have provided the evidence that application exogenous stem cells [mesenchymal stem cells (MSCs) and neural stem cells (NSCs)], not only possess the potential to migrate toward damaged brain tissues but also redeem the damaged cells via differentiation. Besides, MSCs are also known to release anti-inflammatory and growth factors, with potential to improve neurological function.13 Since bone marrow stem cells (BM-MSCs) and umbilical cord mesenchymal stem cells (UC-MSCs) are the most widely applied types of MSCs, clinical studies have illustrated that both BM-MSCs and UC-MSCs administrated via intravenous and lumbar puncture could significantly improve the damaged brain areas.1 However, the lack of targeted and effective therapeutic strategies remains a major challenge for researchers and clinicians. Hence, in the light of these above reported studies, here we aimed to review the previously reported studies to determine the role of stem cells in the context of TBI, to investigate the underlying mechanisms of TBI, to brief about to date available therapeutic strategies, to determine the emerging role of stem cell therapy while discussing the present challenges and future perspective in TBI treatment.
Molecular Mechanisms of TBI
Generally, TBI is regarded as a cascade of acute or reticent mechanical dynamism which ultimately creates a hostile microenvironment by exacerbating inflammation, glial activation, and astrogliosis, which ultimately cause vascular injury and hypoxia resulting in neuronal tissue damage.15,16 Although the recent decade has seen significant advent in the understanding of molecular mechanisms; however, comprehensive grasp on the precise molecular events and related biomarkers remained elusive.17
Biologically, TBI is classified as a primary and secondary injury, wherein the primary injury indicates the direct exposure of mechanical injury that incidentally damage the normal brain function.1 Whereas a secondary lesion is associated with multiplex avalanche events at of molecular and cellular level resulting in death/necrosis of neuronal cells and tissues18, hence indicating the most crucial event in terms of long term management of TBI. Nonetheless, further investigations into secondary damage have revealed neuroinflammation, oxidative damage, reactive oxygen species (ROS) accumulation, cytokine activation, excitotoxicity as prominent mechanistic events that ultimately results in neuronal cell death (Figure 1)
Briefly, excitotoxicity is considered as the most critical even in the biology of TBI particularly in the context of secondary damage. While further investigation into the underlying mechanism indicates that onset of physical injury causes severe damage to the blood-brain barrier (BBB) which instantly enhance the glutamate levels, which persist for about 24 to 48 hours.19 This sustained excitotoxicity and increased glutamate level provoke the spread of depolarization waves in patients.20 Additionally, it has been well established that excitotoxicity manifests a high intracellular concentration of calcium which results in the activation of catabolic enzymes, i.e., phospholipases, proteases, and endonucleases.6 Since phospholipases cause the damage of the cell by disrupting mitochondrial membranes, while protease activation results in DNA fragmentation due to disruption in endonucleases and cell cytoskeleton ultimately resulting in cell death via apoptosis or necrosis6,21, thus providing insight into its mechanism.
Nonetheless, neuroinflammation is another important mechanism manifesting the secondary damage in post-TBI, initiating directly after the onset of primary injury due to the activation of microglial (this event has been indicated as an essential mechanism not only for the reparation of neuronal damage but also to avoid the further damage due to pathogens).22 Further insight into the main molecular mechanism of neuroinflammation exhibits that activation of microglial cells in the brain not only results in disruption of BBB but also elevates the levels of other inflammatory mediators such as cytokines, complementary proteins in brain parenchymal tissues.21 While further studies have reported that the association between neuroinflammation and accumulation of ROS results in activation of crucial cellular events such as DNA oxidation, protein carbonylation, and lipid peroxidation. Eventually, changes in membrane permeability and fluidity occur that enhances enzyme leakage, resulting in caspase-mediated apoptosis.23-26 Hence, suggesting the pivotal role of oxidative stress in secondary damage post-TBI by determining the disparity between antioxidants and ROS.27 Moreover, the activation and upregulation of cell-cycle mediators such as c-myc and cyclins and reduction in the level of cell cycle inhibitors have also been indicated to accelerate the damaging effects of TBI.28
While suppression of caspases and mitochondrial permeabilization in TBI speculate that autophagy could be a leading mechanism that regulates programmed cell death, therefore, it is also regarded as a compensation to inhibit apoptosis.29,30 Hence, the above-mentioned studies speculate that programmed cell death is the dominant mechanism of neuronal cell damage after the incidence of TBI.21 Nonetheless, increasing understanding about the moelcualr events and underlying mechanism has attracted researchers and clinicians to devise more efficient and safe therapeutic strategy for post TBI management.31
Thus collectively suggesting the prerequisite for comprehensive understanding of precise molecular events is the most important steps toward the development of efficient therapeutic strategies.
Role of Stem Cell Therapy in TBI
The ability of MSCs to home at the injured site, differentiate into neuronal cells, and to cross the BBB, make them most promising candidate to aid in therapeutic and restorative functions after TBI.32 Accordingly, Azizi et al.33 have demonstrated that MSCs engrafted to the injured brain site not only survived but also demonstrated the migratory potential similar to NSCs. Besides, another evidence was provided by Kopen et al.34 exhibiting that immuno-depleted MSCs exposure to the lateral ventricle of a neonatal mice model of TBI, results in progression of differentiated progeny of various cells with dermal origin, thus, suggesting MSCs as a valuable tool for treating neurological disorders. While Sanchez-Ramos et al.35 further confirmed that MSCs could potentially differentiate into cells similar to neuronal and glial cells regardless of coculture conditions.35 Another advantage of MSCs is their ability to reside at the site of injury, which could be specifically defined as “their ability being seized into cerebrovascular beamer of a tissue which further mediates the transmigration across the endothelium”36, thus suggesting the ability of MSCs to migrate toward the site of injury.37,38 Further evidence for the effectiveness of early exposure of stem cells after brain injury was provided by Barbash et al.39 where they found that, after 2 days of MSCs infusion, an increased movement of MSCs was observed in the rat’s brain compared to that after 14 days.
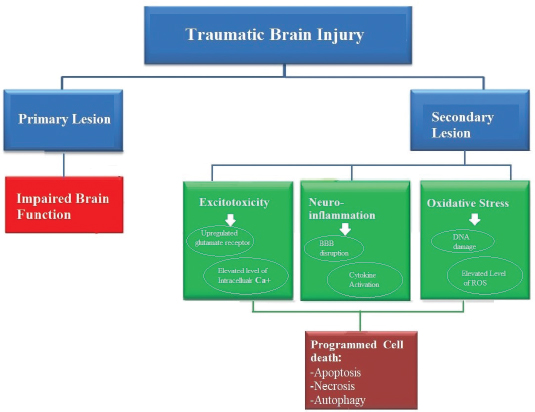
Figure 1. Systematic presentation of major molecular events and their downstream effects underlying TBI. Briefly, TBI is characterized as a primary lesion and secondary lesion, wherein, primary lesion directly results in brain dysfunction, while secondary damage is based on major molecular events i.e., excitotoxicity, neuroinflammation or cytokines activation, oxidative stress, and accumulation of ROS, which eventually results in cell death either by apoptosis, necrosis or autophagy.
Nonetheless, the ability of MSCs to cross the BBB to repair the damage makes it an outstanding neurotherapeutic drug40,41 since damage and disorganisation of BBB is considered as the most critical consequence of TBI.32 Accordingly, Steingen et al.42 have indicated that MSCs inarguably holds the potential to access the endothelial cell barrier of the BBB.43 While Matsushita et al.44 have suggested that the ability of MSCs to pass through the BBB could be attributed to the paracellular pathways in the brain, hence, suggesting MSC’s as the most promising candidate for TBI management.
Additionally, growing evidence has reported the advantage of MSCs application to combat the damaging outcome of TBI.32 For instance, Anbari et al.45 demonstrated that the ability of MSCs to differentiate into neuronal and astrocyte like cells upon transplantation into the rodent model of TBI. Furthermore, it has also been found that differentiation of MSCs leads to a significant increase in neural growth, which further stimulates sensory and motor function improvement.43,45,46
Interestingly, it has also been suggested that the intravenous administration of human Mesenchymal stem cells (hMSCs) derived secretome not only aid in decreasing the apoptosis but also provoke the release of vascular endothelial growth factor (VEGF).47-49 Hence, these findings reinforce the concept that MSCs could potentially aid in neural cell replacement as well in recovery of neural function.50,51 Classically, the regenerative ability of MSCs is attributed to the release of growth factors, i.e., glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, nerve growth factor, and VEGF.32 Besides, its underlying mechanism indicates the ability of MSCs to assist in upregulation in the level of growth factors (Ang-1, VEGF) and microangiogenesis13, which further promote the recovery from TBI. It is also important to mention that the main mechanism through which MSCs aid in damage recovery is through the release of these growth factors that mediate the differentiation of resident.52 Thus suggesting that the administeration of MSCs derived factors solely possess the potential to promote the recovery after the incidence of TBI even in the absence of transplanting cells themselves.32
Preclinical Studies Using MSCs for TBI Treatment
Recently, various animal models and in vitro computational modeling of TBI have contributed to the current understanding of post-traumatic events.53 Therefore, animal modeling remains crucial for a comprehensive understanding of the complex pathophysiological mechanisms correlated with neurological conditions, for evaluating novel therapeutic agents, to ensure the safety of clinical trials, and to predict the degree of success.53
Accumulating evidence from preclinical studies have reported the application of MSCs in TBI models and demonstrated the regenerative potential of MSCs to boost the damage recovery via secreting trophic factors which allow the residing progenitor cells to replace damaged cells and ultimately by the inhibition of inflammatory cascade.54 For instance, a preclinical study has shown that MSCs application can not only reduce the expression of inflammatory molecules but also enhance the reparation of intracranial aneurysms.55
Besides, further evidence from preclinical studies using rodent TBI models have suggested that MSCs can specifically home to the site of injury in the brain tissue but also differentiate into neurons and astrocytes like cells to mend the damaged brain tissue which ultimately aid in the functional revovery.56 Further evidence by Zhang et.al.50 has reported the significant improvement and prominent decrease in brain water content of rat model of TBI after transplantation of MSCs.50 Besides, they also reported that MSCs application of MSCs can significantly reduce the neuro-inflammation in the injured cortex region of rodent brain by decreasing the proliferation of macrophages, microglia, neutrophils, apoptotic cells, and reducing the level of proinflammatory cytokines.50 Similarly, Guo et al.57 used TBI mice model to demonstrate that MSCs transplantation could significantly aid in the recovery of the neurological function, amend the memory and learning ability while decreasing the neuronal apoptosis.57 The fact that rodent models are the most frequently used animals to represent different injury mechanisms associated with human TBI in preclinical trials; however, higher species with the anatomical and functional similarity of the brain with the human being could bring a better outcome for translation to clinical trials. Therefore, before the initiation of clinical trials, an effective treatment in rodents should be tested by confirming its efficacy in large animal models that could closely emulate the complex pathogenesis of TBI in humans.53 For instance, the application of stem cell therapy in the swine model of TBI demonstrated that early intervention of only a sole dosage of MSC-derived exosomes preeminently abate the size of brain injury as well as reduce the swelling in lesion area which could be attributed to the reduction of inflammatory response and ultimately results in reduction in blood-based cerebral biomarkers to restore the integrity of BBB.58 Despite these above-mentioned advantages, a comprehensive understanding of animal behavior, as well as the deep knowledge about the undergoing molecular mechanisms, are required to devise an effective strategy for the translation of these results from preclinical into clinical trials.
Clinical Trials Using Stem Cells for TBI Treatment
Successful resulting data from preclinical studies have largely inspired scientists and clinicians to evaluate the clinical efficacy of MSCs transplantation. However, less than 4% of intravenously injected MSCs could reach the arterial circulation.59 Although no adverse events have been illustrated regarding the isolation or transplantation of MSCs, yet, dose-related pulmonary toxicity remained an the major concern upon administrating a dose of < 9 × 106 cells/kg.60 Thus indicating a rate limiting aspect that should be overcome in future trials assessing efficacy. Besides lumbar puncture and intracerebral transplantation of MSCs have not exhibited any unfavorable events during isolation and transplantation of autologous MSCs.61,62 Although intravenous administration of BM-MSCs has been attributed to the improved recovery and reduction in systemic inflammatory markers in the blood.60,63 Yet, its application could not achieve any prominent or successful outcome in context of functional recovery after the application of autologous BM-MSCs therapy via lumbar puncture in TBI patients.62
Besides, MSC’s isolated from other sources including human umbilical cord blood (hUCB-MSCs) have also been widely implicated in MSC-based therapies. For instance, recently a clinical trial investigating the role of allogeneic hUCB-MSCs in the recovery of TBI sequelae, randomly recruited 40 patients to treat with hUCB-MSCs via lumbar puncture.64 Intriguingly, their data indicated that allogeneic administration of hUCB-MSC via lumbar injection could also be a safe strategy to attenuate the chronic motor disability in TBI patients.65 Hence, providing a basis for future researchfor application of hUCB in TBI management.64
Nonetheless, despite encouraging outcomes from preclinical and clinical trials based on complex pathophysiology and molecular mechanism of TBI, in context for the application of stem cell therapy in its treatment, there is still great room left for the improvement in therapeutic strategy in terms of its efficacy and safety which can promote the functional recovery of brain tissue. The major factor could be the deficit ability of mammalian neuronal tissue to regenerate itself which ultimately appeal to the external therapeutic assistance.
Overcoming Clinical Challenges
It has been indicated that clinical trial studies have encountered little success due to inappropriate administration methods. Yet there is no common standard for the assessment of outcome measures. Thus, overcoming the lack of an appropriate method of administration and optimal timing of stem cell delivery by using clinically effective methods remains the leading challenge in TBI for the neuroscience research community as well as for the pharmaceutical and drug development industry.53 Although an increasing amount of research has been organized to determine the effective routes for stem cell delivery i.e., direct route, intravenous, intracerebral, and intra-arterial route; however, these methods exhibit their pros and cons. For instance, despite being the most convenient method to approach the circulatory system66,67, the first-pass pulmonary sequestration is considered as major shortcoming of intravenous application.39,68 Whereas, intra-arterial transplantation hold the advantage of local induction of stem cells via circumventing the high pulmonary first-pass effect.69 While intracerebrally transplanted stem cells have demonstrated the significant migrating ability toward the injured site, which could be attributed to enhanced load of the stem cell at the site of disease/injury.70 Accordingly, a study by Xiong et al.71 has demonstrated that intranasal (i.n.) transplantation of MSC via nasal mucosa either by cribriform plate permeation or via uptake by the olfactory pathway to the tissues in the brain72, offers the most invasive and effective route. While Danielyan et al.73 further provided the evidence for the presence fluorescently labeled MSCs in the brain tissues even after 1 hour of i.n. administration in a rodent TBI model. Nonetheless, Galeano et al.74 indicated that ensnarement of cells in the nasal cavity is the major drawback of this procedure. Thus suggesting the requirement of effective safe and minimally invasive delivery method.37
Nonetheless, recently reported studies have illustrated the crucial role of exosomes derived from MSCs in augmenting their therapeutic efficacy.75,76 Exosomes have been indicated to77 play a vital role in intercellular communication.78 A shred of fascinating evidence has illustrated that MSC-derived exosomes hold similar therapeutic efficiency as their parent MSC.71,79,80 Concordinlgy, Zhang et al.76 has shown that i.v. injection of exosomes could effectively improve the functional recovery in rats after TBI, yet their remedial effects are attributed to the cultural condition of parent cells i.e., 2D versus 3D81, and their cargo i.e., mRNA, miRNA, lncRNA, mitochondrial DNA, and protein.71 Collectively, these properties make the application of exosomes as an appealing therapeutic strategy as compared to their parent MSCs.82 In addition to exosome based therapy, recent studies have also indicated the application of genetically modified MSCs to produce cytokines, chemokines, and soluble growth factors.49 Peculiarly, growth factors have been reported to remarkably enhance the survival of stem cells as well as neuronal cells by aiding in neurogenesis and angiogenesis process at the site of injury.45 Although these novel strategies are still under investigation, yet they provide an attractive and effective source that can not only overcome the cell administration issue but also provide an alternative acelluar therapeutic strategies to combat the effects of TBI.
Conclusion
Collectively, this review suggests that several factors, i.e., scarce understanding about the route of cell delivery, lack of understanding about after effects and potential complications, obscure ethical concern particularly due to lack of clinical data, as the major hurdle in clinical application of regenerative medicine for TBI. Thus keeping in view, the scarce clinical trails using stem cells for TBI treatment, multicenter set of future study aiming at randomized prospective is prerequisite, which could potentially suggest the suitable future therapeutic modality for TBI. Hence, comprehensive knowlegde about the role of underlying mechanism combined with translation of these novel findings could aid in the development of new therapeutic strategies for TBI.
Limitations of the study
Due to scarce data from relevant clinical studies, the present review does not encompass the detailed clinical outcome. However, a brief revelation review about the current situation and possible strategies would be interesting for research scientists and clinicians to design their further studies by keeping in view the limitations and advantages of to-date available strategies.
Acknowledgement
Not applicable.
List of Abbreviations
| BBB | Blood brain barrier |
| BM-MSCs | Bone marrow stem cells |
| GCS | Glasgow Coma Scale |
| hUCB-MSCs | Human umbilical cord blood |
| MSCs | Mesenchymal stem cells |
| NSCs | Neural stem cells |
| TBI | Traumatic brain injury |
| UC-MSCs | Umbilical cord mesenchymal stem cells |
| VEGF | Vascular endothelial growth factor |
Grant support & financial disclosure
Not applicable.
Conflict of interest
None to declare.
Ethical approval
Not applicable.
Authors’ contribution
LC, YS: Contributed equally as first co-authors of this article)
LC, YS, YJ: Conception and design of the review, drafting the manuscript, Acquistion of data, Critical review of manuscript for important intellectual input.
HL: Acquisition of data, drafting of figures in the manuscript.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Authors’ details
Cui Lianxu1, Yasmeen Saeed2, Li Haomin1, Yang Jingli3
- Department of Neurosurgery, The First People’s Hospital of Foshan, Foshan, China
- Department of scientific research and project development Guangdong VitaLife Biotechnology Co., LTD, Foshan, China
- School of Medicine, Foshan University, Foshan, China
References
- Schepici G, Silvestro S, Bramanti P, Mazzon E. Traumatic brain injury and stem cells: an overview of clinical trials, the current treatments and future therapeutic approaches. Medicina (Kaunas). 2020;56(3):137. https://doi.org/10.3390/medicina56030137
- Arciniegas DB, Held K, Wagner P. Cognitive impairment following traumatic brain injury. Curr Treat Options Neurol. 2002;4(1):43–57. https://doi.org/10.1007/s11940-002-0004-6
- Irrera N, Pizzino G, Calò M, Pallio G, Mannino F, Famà F, et al. Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front Pharmacol. 2017;14(8):459–71. https://doi.org/10.3389/fphar.2017.00459
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–41. https://doi.org/10.1016/S1474-4422(08)70164-9
- Meaney DF, Morrison B, Dale Bass C. The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J Biomech Eng. 2014;136(2):021008. https://doi.org/10.1115/1.4026364
- Wang K, Cui D, Gao L. Traumatic brain injury: a review of characteristics, molecular basis and management. Front Biosci (Landmark Ed). 2016;21(1):890–9. https://doi.org/10.2741/4426
- Cole TB. Global road safety crisis remedy sought: 1.2 million killed, 50 million injured annually. JAMA. 2004;291(21):2531–2. https://doi.org/10.1001/jama.291.21.2531
- Reis C, Wang Y, Akyol O, Ho WM, Ii RA, Stier G, et al. What’s new in traumatic brain injury: update on tracking, monitoring and treatment. Int J Mol Sci. 2015;16(6):11903–65. https://doi.org/10.3390/ijms160611903
- Menon DK, Maas AI. Traumatic brain injury in 2014. Progress, failures and new approaches for TBI research. Nat Rev Neurol. 2015;11(2):71–2. https://doi.org/10.1038/nrneurol.2014.261
- Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014;13(8):844–54. https://doi.org/10.1016/S1474-4422(14)70120-6
- Bodanapally UK, Sours C, Zhuo J, Shanmuganathan K. Imaging of traumatic brain injury. Radiol Clin North Am. 2015;53(4):695–715. https://doi.org/10.1016/j.rcl.2015.02.011
- Picetti E, Rossi S, Abu-Zidan FM, Ansaloni L, Armonda R, Baiocchi GL, et al. WSES consensus conference guidelines: monitoring and management of severe adult traumatic brain injury patients with polytrauma in the first 24 hours. World J Emerg Surg. 2019;14(11):53. https://doi.org/10.1186/s13017-019-0270-1
- Zhou Y, Shao A, Xu W, Wu H, Deng Y. Advance of stem cell treatment for traumatic brain injury. Front Cell Neurosci. 2019;13(13):301–9. https://doi.org/10.3389/fncel.2019.00301
- Young L, Rule GT, Bocchieri RT, Walilko TJ, Burns JM, Ling G. When physics meets biology: low and high-velocity penetration, blunt impact, and blast injuries to the brain. Front Neurol. 2015;6(7):89. https://doi.org/10.3389/fneur.2015.00089
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33(31):12870–86. https://doi.org/10.1523/JNEUROSCI.2121-13.2013
- Englander J, Cifu DX, Diaz-Arrastia R. Information/education page. Seizures and traumatic brain injury. Arch Phys Med Rehabil. 2014;95(6):1223–4. https://doi.org/10.1016/j.apmr.2013.06.002
- Veenith T, Goon SS, Burnstein RM. Molecular mechanisms of traumatic brain injury: the missing link in management. World J Emerg Surg. 2009;4(2):7. https://doi.org/10.1186/1749-7922-4-7
- Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic brain injury: current treatment strategies and future endeavors. Cell Transplant. 2017;26(7):1118–30. https://doi.org/10.1177/0963689717714102
- Tasker RC. Spreading depolarisations and traumatic brain injury: time course and mechanisms. Lancet Neurol. 2012;11(5):389–90. https://doi.org/10.1016/S1474-4422(12)70084-4
- Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J Neurotrauma. 2016;33(19):1775–83. https://doi.org/10.1089/neu.2015.4226
- Ladak AA, Enam SA, Ibrahim MT. A review of the molecular mechanisms of traumatic brain injury. World Neurosurg. 2019;131:126–32. https://doi.org/10.1016/j.wneu.2019.07.039
- Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res Brain Res Rev. 2005;48(2):388–99. https://doi.org/10.1016/j.brainresrev.2004.12.028
- Mutinati M, Pantaleo M, Roncetti M, Piccinno M, Rizzo A, Sciorsci RL. Oxidative stress in neonatology: a review. Reprod Domest Anim. 2014;49(1):7–16. https://doi.org/10.1111/rda.12230
- Wang JW, Wang HD, Cong ZX, Zhou XM, Xu JG, Jia Y, et al. Puerarin ameliorates oxidative stress in a rodent model of traumatic brain injury. J Surg Res. 2014;186(1):328–37. https://doi.org/10.1016/j.jss.2013.08.027
- Cheng G, Kong RH, Zhang LM, Zhang JN. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br J Pharmacol. 2012;167(4):699–719. https://doi.org/10.1111/j.1476-5381.2012.02025.x
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. https://doi.org/10.1007/s00401-009-0619-8
- Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: a double-edged sword revisited. Annu Rev Pathol. 2014;9:119–45. https://doi.org/10.1146/annurev-pathol-012513-104651
- Stoica BA, Byrnes KR, Faden AI. Cell cycle activation and CNS injury. Neurotox Res. 2009;16(3):221–37. https://doi.org/10.1007/s12640-009-9050-0
- Bredesen DE. Programmed cell death mechanisms in neurological disease. Curr Mol Med. 2008;8(3):173–86. https://doi.org/10.2174/156652408784221315
- Bayly PV, Dikranian KT, Black EE, Young C, Qin YQ, Labruyere J, et al. Spatiotemporal evolution of apoptotic neurodegeneration following traumatic injury to the developing rat brain. Brain Res. 2006;1107(1):70–81. https://doi.org/10.1016/j.brainres.2006.05.102
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–57. https://doi.org/10.1089/089771502753754037
- Hasan A, Deeb G, Rahal R, Atwi K, Mondello S, Marei HE, et al. Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol. 2017;8(4):28. https://doi.org/10.3389/fneur.2017.00028
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95(7):3908–13. https://doi.org/10.1073/pnas.95.7.3908
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96(19):10711–6. https://doi.org/10.1073/pnas.96.19.10711
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164(2):247–56. https://doi.org/10.1006/exnr.2000.7389
- Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–16. https://doi.org/10.1016/j.stem.2009.02.001
- Walker PA, Shah SK, Harting MT, Cox CS Jr. Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Dis Model Mech. 2009;2(1-2):23–38. https://doi.org/10.1242/dmm.001198
- Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J Neurosurg. 2006;104(2):272–7. https://doi.org/10.3171/jns.2006.104.2.272
- Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–8. https://doi.org/10.1161/01.CIR.0000084828.50310.6A
- Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2(3):106–13. https://doi.org/10.1016/1357-4310(96)88720-X
- Gaillard PJ, Visser CC, Appeldoorn CC, Rip J. Targeted blood-to-brain drug delivery -10 key development criteria. Curr Pharm Biotechnol. 2012;13(12):2328–39. https://doi.org/10.2174/138920112803341815
- Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44(6):1072–84. https://doi.org/10.1016/j.yjmcc.2008.03.010
- Zanier ER, Pischiutta F, Riganti L, Marchesi F, Turola E, Fumagalli S, et al. Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurotherapeutics. 2014;11(3):679–95. https://doi.org/10.1007/s13311-014-0277-y
- Matsushita T, Kibayashi T, Katayama T, Yamashita Y, Suzuki S, Kawamata J, et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502(1):41–5. https://doi.org/10.1016/j.neulet.2011.07.021
- Anbari F, Khalili MA, Bahrami AR, Khoradmehr A, Sadeghian F, Fesahat F, et al. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen Res. 2014;9(9):919–23. https://doi.org/10.4103/1673-5374.133133
- Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, Pischiutta F, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39(11):2501–10. https://doi.org/10.1097/CCM.0b013e31822629ba
- Chuang TJ, Lin KC, Chio CC, Wang CC, Chang CP, Kuo JR. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J Trauma Acute Care Surg. 2012;73(5):1161–7. https://doi.org/10.1097/TA.0b013e318265d128
- Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond). 2013;124(3):165–76. https://doi.org/10.1042/CS20120226
- Azari MF, Mathias L, Ozturk E, Cram DS, Boyd RL, Petratos S. Mesenchymal stem cells for treatment of CNS injury. Curr Neuropharmacol. 2010;8(4):316–23. https://doi.org/10.2174/157015910793358204
- Zhang R, Liu Y, Yan K, Chen L, Chen XR, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10(1):106. https://doi.org/10.1186/1742-2094-10-106
- Grigorian AS, Gilerovich EG, Pavlichenko NN, Kruglyakov PV, Sokolova IB, Polyntsev DG. Effect of transplantation of mesenchymal stem cells on neuronal survival and formation of a glial scar in the brain of rats with severe traumatic brain injury. Bull Exp Biol Med. 2011;150(4):551–5. https://doi.org/10.1007/s10517-011-1187-1
- Galindo LT, Filippo TR, Semedo P, Ariza CB, Moreira CM, Camara NO, et al. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. 2011;2011:564089. https://doi.org/10.1155/2011/564089
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. 2013;14(2):128–42. https://doi.org/10.1038/nrn3407
- Dobrowolski S, Ebner F, Lepski G, Tatagiba M. Foramen magnum meningioma: the midline suboccipital subtonsillar approach. Clin Neurol Neurosurg. 2016;145:28–34. https://doi.org/10.1016/j.clineuro.2016.02.027
- Adibi A, Sen A, Mitha AP. Cell therapy for intracranial aneurysms: a review. World Neurosurg. 2016;86(4):390–8. https://doi.org/10.1016/j.wneu.2015.10.082
- Wang S, Kan Q, Sun Y, Han R, Zhang G, Peng T, et al. Caveolin-1 regulates neural differentiation of rat bone mesenchymal stem cells into neurons by modulating Notch signaling. Int J Dev Neurosci. 2013;31(1):30–5. https://doi.org/10.1016/j.ijdevneu.2012.09.004
- Guo S, Zhen Y, Wang A. Transplantation of bone mesenchymal stem cells promotes angiogenesis and improves neurological function after traumatic brain injury in mouse. Neuropsychiatr Dis Treat. 2017;13(6):2757–65. https://doi.org/10.2147/NDT.S141534
- Williams AM, Bhatti UF, Brown JF, Biesterveld BE, Kathawate RG, Graham NJ, et al. Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg. 2020;88(2):207–18. https://doi.org/10.1097/TA.0000000000002563
- Margulies S, Anderson G, Atif F, Badaut J, Clark R, Empey P, et al. Combination therapies for traumatic brain injury: retrospective considerations. J Neurotrauma. 2016;33(1):101–12. https://doi.org/10.1089/neu.2014.3855
- Cox CS Jr, Hetz RA, Liao GP, Aertker BM, Ewing-Cobbs L, Juranek J, et al. Treatment of Severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells. 2017;35(4):1065–79. https://doi.org/10.1002/stem.2538
- Zhang ZX, Guan LX, Zhang K, Zhang Q, Dai LJ. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy. 2008;10(2):134–9. https://doi.org/10.1080/14653240701883061
- Tian C, Wang X, Wang X, Wang L, Wang X, Wu S, et al. Autologous bone marrow mesenchymal stem cell therapy in the subacute stage of traumatic brain injury by lumbar puncture. Exp Clin Transplant. 2013;11(2):176–81. https://doi.org/10.6002/ect.2012.0053
- Cox CS Jr, Baumgartner JE, Harting MT, Worth LL, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68(3):588–600. https://doi.org/10.1227/NEU.0b013e318207734c
- Chrostek MR, Fellows EG, Guo WL, Swanson WJ, Crane AT, Cheeran MC, et al. Efficacy of cell-based therapies for traumatic brain injuries. Brain Sci. 2019;9(10):270. https://doi.org/10.3390/brainsci9100270
- Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84. https://doi.org/10.1016/j.brainres.2013.08.001
- Allers C, Sierralta WD, Neubauer S, Rivera F, Minguell JJ, Conget PA. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 2004;78(4):503–8. https://doi.org/10.1097/01.TP.0000128334.93343.B3
- Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. https://doi.org/10.1159/000047856
- Tolar J, O’shaughnessy MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell S, Riddle M, et al. Host factors that impact the biodistribution and persistence of multipotent adult progenitor cells. Blood. 2006;107(10):4182–8. https://doi.org/10.1182/blood-2005-08-3289
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56(12):1666–72. https://doi.org/10.1212/WNL.56.12.1666
- Mahmood A, Lu D, Yi L, Chen JL, Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J Neurosurg. 2001;94(4):589–95. https://doi.org/10.3171/jns.2001.94.4.0589
- Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. 2017;12(1):19–22. https://doi.org/10.4103/1673-5374.198966
- Li G, Bonamici N, Dey M, Lesniak MS, Balyasnikova IV. Intranasal delivery of stem cell-based therapies for the treatment of brain malignancies. Expert Opin Drug Deliv. 2018;15(2):163–72. https://doi.org/10.1080/17425247.2018.1378642
- Danielyan L, Schäfer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88(6):315–24. https://doi.org/10.1016/j.ejcb.2009.02.001
- Galeano C, Qiu Z, Mishra A, Farnsworth SL, Hemmi JJ, Moreira A, et al. The route by which intranasally delivered stem cells enter the central nervous system. Cell Transplant. 2018;27(3):501–14. https://doi.org/10.1177/0963689718754561
- Belting M, Christianson HC. Role of exosomes and microvesicles in hypoxia-associated tumour development and cardiovascular disease. J Intern Med. 2015;278(3):251–63. https://doi.org/10.1111/joim.12393
- Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–67. https://doi.org/10.3171/2014.11.JNS14770
- Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–8. https://doi.org/10.1016/j.bbagen.2012.03.017
- Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83(11):1484–94. https://doi.org/10.1016/j.bcp.2011.12.037
- Zhao Y, Gibb SL, Zhao J, Moore AN, Hylin MJ, Menge T, et al. Wnt3a, a protein secreted by mesenchymal stem cells is neuroprotective and promotes neurocognitive recovery following traumatic brain injury. Stem Cells. 2016;34(5):1263–72. https://doi.org/10.1002/stem.2310
- Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, et al. Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med. 2015;4(10):1131–43. https://doi.org/10.5966/sctm.2015-0078
- Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, et al. Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69–81. https://doi.org/10.1016/j.neuint.2016.08.003
- Das M, Mayilsamy K, Mohapatra SS, Mohapatra S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. 2019;30(8):839–55. https://doi.org/10.1515/revneuro-2019-0002
