Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(2):123-129
ORIGINAL ARTICLE
Effects of Ribavirin on thickness of testicular capsule in albino rats
Alvia Batool1*, Maryam Fatima1, Fozia Farzana2
Received: 19 November 2020 Revised date: 08 May 2021 Accepted: 29 May 2021
Correspondence to: Alvia Batool
*Anatomy Department, Fatima Memorial Hospital College of Medicine & Dentistry, Lahore, Pakistan.
Email: alvia.batool@gmail.com
Full list of author information is available at the end of the article.
ABSTRACT
Background & Objective:
Ribavirin (RBV) due to its mutagenic property exerts cytotoxic effects on the testicular seminiferous tubules and its interstitium in various experimental animals. However, no study was done on testicular capsule. Therefore, the present experimental study is designed to observe the transient effects of RBV in different doses on the testicular capsule thickness in albino rats.
Methods:
A total of 72 sexually mature adult male albino rats were divided into four groups: A (control) and, B, C, D where RBV was administered intraperitoneally for 5 days in different doses (20, 100, and 200 mg/kg body weight, respectively). Group A received distilled water intraperitoneally. Each group was further divided into three subgroups according to three sacrificial time points that were 20th, 40th, and 60th day from the last exposure to drug. Randomly selected rats from each group were sacrificed on every time point. Histological slides were prepared and changes were observed microscopically.
Results:
On 20th sacrificial day, the increase in the thickness of capsules was seen in all groups with subcapsular edema in comparison to control group (p < 0.001). After discontinuation of drug, histological evidence of recovery in the form of decrease in the thickness of capsule with decrease in subcapsular edema was observed in low dose groups on 40th and 60th sacrificial days as compared to high dose groups which showed less recovery on both time points (p < 0.001).
Conclusion:
The testicular capsular thickening induced by RBV is reversible after cessation of treatment.
Keywords:
Testes, Rat, Cytotoxicity, Capsule, Thickness, Ribavirin.
Introduction
Hepatitis is a great community health problem in the world which is related to high death rate due to liver disease [1]. The viruses, parasites, drugs, some toxic agents, and certain autoimmune reactions against liver cells lead to hepatitis. Hepatitis C virus found in 1989 is spread by exposure to infected blood; 60%-80% of long-standing infections bring about liver cirrhosis and hepatic cancer [2]. Ribavirin (RBV) orally and injectable Interferon alpha adjunctive medication has been proved as a potent therapy for chronic Hepatitis C. RBV, an antiviral medication was made in 1970 and its wide range action against viruses was highlighted in 1972 [1]. Its aerosol type has been accepted as a remedy for Respiratory Syncytial Virus in youngsters and Intravenous therapy decreases death rate from Lassa and hemorrhagic fevers [3]. It is a purine (guanosine) nucleoside equivalent with modified base and D-ribose sugar having chemical name 1-beta-D-ribofurnosyl-1H-1, 2, 4-triazole-3-carboxamide. Phosphorylation of RBV occurs inside the cell by host cell enzymes. RBV exists in three metabolic forms like Mono-, Di- and Triphosphates, they possess the ability to protect from many viruses. RBV-5’-triphosphate is the main form that is found intracellularly [1,3,4]. RBV triphosphate ceases the duplication of various DNA and RNA viruses due to its broad-spectrum antiviral action. It may interfere with the synthesis of guanosine triphosphate (GTP); it can prevent capping of viral messenger RNA or it may stop the viral RNA-dependent polymerase of certain viruses [5].
RBV is toxic to the embryos of laboratory animals. Patients (male and female) using this drug should avoid conception during the treatment and for at least 6 months thereafter [3-5]. Intraperitoneal injection of RBV affects the testes after it gets absorbed from the peritoneal cavity. It reaches the germ cells and exerts its mutagenicity. It prevents the action of Inosine monophosphate dehydrogenase that probably decreases GTP concentration inside the cells. In this way, RBV is mutagenic for many viruses [6].
RBV is documented for the production of structural and functional disturbances in the tissues like bone marrow, epididymis and testis of various experimental animals [7,8].
RBV seems to play its cytotoxic role by causing cell death and blockage of cell division [8]. In patients of Crimean-Congo hemorrhagic fever getting prescribed doses of this drug, it was proved toxic to genetic material in transient way because of its metabolic products [9].
RBV is not allowed for either sex during 6 months prior to conception and its use is also prohibited during pregnancy due to its teratogenicity. Pregnancy registry of RBV was founded in 2003 for documentation of its most probable embryotoxicity [10]. Semen aberrations were noted in patients who were under treatment of adjunctive medication containing Pegylated interferons and RBV for chronic hepatitis C [11].
The cytotoxicity of RBV has been noted in seminiferous tubules, sperms, and interstitium of testes in previous studies but no one had highlighted the effects of this drug on testicular capsule. The present study is, therefore, designed to observe the changes in testicular capsule of rats as an experimental model treated with RBV and to observe reversibility of these changes after discontinuation of drug.
Methods
This randomized controlled experimental study was conducted at Postgraduate Medical Institute Lahore, Pakistan after approval from the Ethical Review Committee of University of Health Sciences, Lahore. Seventy-two sexually mature adult male Wistar albino rats, weighing approximately 180-200 g were obtained from the animal house of National Institute of Health, Islamabad. The animals were examined completely and weighed before starting the exper-iment. The rats were housed at the animal house under optimum conditions of temperature 24°C ± 2°C, humidity 50% ± 10%, and in light and dark cycles of 12 hours. All ani-mals were given a standard pallet diet and were provided with water ad libitum. After adaptation of 5 days, the rats were randomly divided into four groups A, B, C, and D with the help of random number table. Each group had 18 rats. Commercially prepared RBV (Getz Pharma, Karachi, Pakistan) was weighed on a scientific balance (Sartorius pre-cision balance®, Germany), dissolved in distilled water and was administered intraperitoneally once daily at 24 hours. interval for five successive days in doses of 20, 100, and 200 mg/kg to the experimental animals of group B, group C, and group D, respectively, whereas control group A was given equal amounts of distilled water with the same route at the same time interval and for the same duration. At the 20th, 40th, and 60th day, after the last exposure to the drug, six animals were randomly selected and sacrificed from each study group including control group. Three subgroups of each study group, A, B, C, and D were formed according to three sacrificial times, hence making 12 subgroups in total (Table 1).
Rats were anaesthetized by giving chloroform and they were sacrificed. A vertical midline incision was given that was prolonged side ways to expose abdomen and thorax. Scrotum was incised vertically; epididymis was removed from testes and both testes were taken out. Weight of each testis was recorded separately. Testes were fixed in Bouin’s solution for 18 hours. The tissues were processed and blocks were prepared. Horizontal sections of 3-5 μm thickness were made with the help of a microtome. The slides were stained with Hematoxylin & Eosin. Micrometry of testicular capsules of all study groups, was done using an ocular micrometer under ×100 magnification. Changes in thickness of testicular capsules were noted in comparison with controls and observations were recorded in tables for all study groups at various sacrificial times.
Table 1. Experimental chart.
| Groups | Subgroups | Dose of RBV | Schedule of sacrifice days from the last dose |
|---|---|---|---|
| Control A | A1 | 0.75 ml distilled water | A1, 20th day |
| A2 | A2, 40th day | ||
| A3 | A3, 60th day | ||
| Experimental B | B1 | 20 mg/kg RBV dissolved in 0.75 ml distilled water. | B1, 20th day |
| B2 | B2, 40th day | ||
| B3 | B3, 60th day | ||
| Experimental C | C1 | 100 mg/kg RBV dissolved in 0.75 ml distilled water. | C1, 20th day |
| C2 | C2, 40th day | ||
| C3 | C3, 60th day | ||
| Experimental D | D1 | 200 mg/kg RBV dissolved in 0.75 ml distilled water. | D1, 20th day |
| D2 | D2, 40th day | ||
| D3 | D3, 60th day |
Statistical analysis
The data was analyzed by using Statistical Package for Social Sciences 23.0. For quantitative variables Mean (± SD) was calculated. Analysis of variance (ANOVA) was applied to show the statistical difference in the mean of all groups. Post-hoc Tukey test was applied to evaluate the difference of means between the groups at 5% level of significance (p ≤ 0.05).
Results
Histological examination revealed that the testes of control group were covered by a dense connective tissue capsule called tunica albuginea. Nuclei of fibroblasts were seen dispersed among collagen fibers. Blood vessels of tunica vasculosa were also observed next to the capsule (Figures 1-3). The structure of the capsule was normal in all experimental groups but the thickness of capsule was found to be increasing with the increase in the dose at various sacrificial times. Subcapsular edema and vascular congestion was also noted in all experimental groups. Results showed an increase in the thickness of capsule on 20th day of sacrifice in all experimental groups as compared to control group A1 (Figure 4). The blood vessels near the capsule were found dilated, contain-ing red blood cells (RBCs). Pinkish material present next to the capsule was considered subcapsular edema fluid. It was also observed on the first two sacrifice times in all experimental groups (Table 2 and Figures 1-3). Overall increase in the values was compared by applying ANOVA, that was significant with p < 0.001. Post hoc test showed statistically significant differences between all the study groups. The test revealed no significant difference between C1 and D1 groups (Table 2).
The gradual reduction of thickness of capsule was observed on 40th as compared to the mean values observed on 20th day as the effect of drug is reducing (Figure 4). Overall reduction in the values was compared by applying ANOVA that was significant with p < 0.001. Post hoc test showed statistically significant differences between all study groups (Table 2).
The mean values of capsular thickness showed more reduction in low dose group than high dose experimental groups, on the 60th day from the last dose as compared to the mean values on 40th day as there is more reduction in drug effects (Figure 4). This reduction in the values was compared by applying ANOVA, that was significant with p < 0.001. Therefore, post hoc test was applied, that showed statistically signifi-cant differences between all study groups (Table 2). On 60th days of sacrifice capsule was thin in group B3 (Figure 3), but it remained thick in C3 and D3 experimental groups when compared with control group A3 (Figure 3 and Table 2). Although some blood vessels were containing RBCs in group B3, there was no subcapsular edema fluid (Figure 3). Group C3 and D3 were showing the same findings of dilated vessels containing RBCs and subcapsular edema fluid accumulation as seen on the first two sacrifice times (Figure 3 and Table 2).
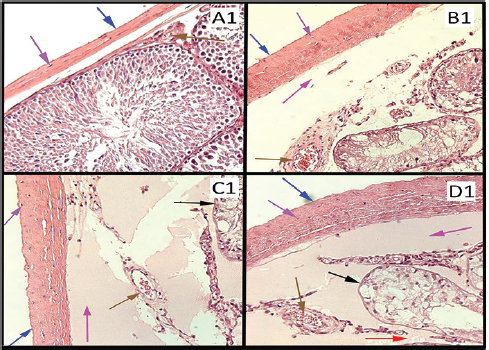
Figure 1. (A1) Photomicrograph of rat’s testis of control group on 20th day labeled with colored arrows, showing capsule (Blue) with fibroblast nuclei (Purple). Blood vessel (Brown) Seminiferous tubule with germ cells (Black). Interstitium (Red). H&E stain ×100. (B1, C1, and D1) Photomicrographs of rat’s testes of low dose, medium dose, and high dose groups on 20th day labeled with colored arrows, showing thick capsules (Blue) more in C1 and D1, fibroblast nuclei (Purple). Subcapsular edema (Magenta). Blood vessel with RBCs (Brown). Shrunken Seminiferous tubules with degenerating germ cells (Black). Interstitium widened and edematous (Red). H&E stain ×100.
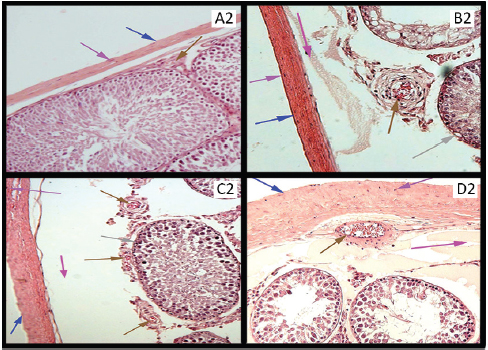
Figure 2. (A2) Photomicrograph of rat’s testis of control group on 40th day labeled with colored arrows, showing capsule (Blue) with fibroblast nuclei (Purple). Blood vessel (Brown) Seminiferous tubule with germ cells (Black). Interstitium (Red). H&E stain ×100. (B2, C2, and D2) Photomicrographs of rat’s testes of low dose, medium dose, and high dose groups on 40th day labeled with colored arrows, showing thick capsules (Blue)with fibroblast nuclei (Purple). Subcapsular edema (Magenta). Blood vessels with RBCs (Brown). Seminiferous tubules with regeneration in germinal epithelium (Gray). Widened and edematous interstitium (Red). H&E stain ×100.
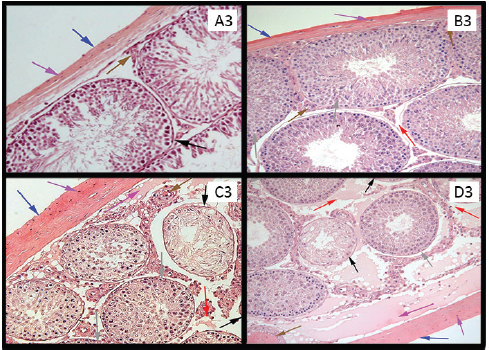
Figure 3. (A3) Photomicrograph of rat’s testis of control group on 60th day, labeled with colored arrows showing capsule (Blue) with fibroblast nuclei (Purple). Blood vessel (Brown) Seminiferous tubule with germ cells (Black). Interstitium (Red). H&E stain. ×100. (B3) A Photomicrograph of rat’s testis of low dose group labeled with colored arrows, showing thin capsule (Blue) with fibroblast nuclei (Purple) and no subcapsular edema. Blood vessel (Brown). Seminiferous tubules with regeneration of germinal epithelium (Gray). Interstitium with no widening and edema (Red). H&E stain ×100. (C3 and D3) Photomicrographs of rat’s testes of medium dose & high dose groups labeled with colored arrows, showing thick capsules (Blue) with fibroblast nuclei (Purple) and subcapsular edema (Magenta). Blood vessel with RBCs (Brown). Shrunken seminiferous tubules with degeneration of germinal epithelium (Black). Seminiferous tubules with regeneration in germ cells (Gray). Widened and edematous interstitium (Red). H&E stain ×100.
Table 2. Comparison of mean values of capsular thickness (micrometer) in study groups at different sacrifice times.
| Times | Groups | Capsular thickness μm | ANOVA | Post hoc test | |
|---|---|---|---|---|---|
| Mean ± SD | P-value | Comparison groups | P-value | ||
| 20th day | A1 | 25 ± 5.48 | <0.001** | A1-B1 | <0.001** |
| B1 | 75 ± 5.48 | A1-C1 | <0.001** | ||
| C1 | 93.33 ± 5.16 | A1-D1 | <0.001** | ||
| D1 | 95 ± 5.48 | B1-C1 | <0.001** | ||
| B1-D1 | <0.001** | ||||
| C1-D1 | 0.950 | ||||
| 40th day | A2 | 26.6 ± 5.16 | <0.001** | A2-B2 | <0.001** |
| B2 | 70.00 ± 6.32 | A2-C2 | <0.001** | ||
| C2 | 81.67 ± 4.08 | A2-D2 | <0.001** | ||
| D2 | 96.67 ± 5.16 | B2-C2 | 0.005** | ||
| B2-D2 | <0.001** | ||||
| C2-D2 | <0.001** | ||||
| 60th day | A3 | 23.33 ±5.16 | <0.001** | A3-B3 | <0.001** |
| B3 | 41.67 ±7.53 | A3-C3 | <0.001** | ||
| C3 | 63.33 ± 5.16 | A3-D3 | <0.001** | ||
| D3 | 75.00 ± 5.48 | B3-C3 | <0.001** | ||
| B3-D3 | <0.001** | ||||
| C3-D3 | 0.013* | ||||
SD = Standard deviation.
Discussion
In this study, it was observed that exposure to RBV in all experimental groups at all sacrificial times led to a change in thickness of testicular capsule with congested vessels of capsule and subcapsular edema. This change was statistically significant in all experimental groups when comparison was made with control groups at all time points (p < 0.001, Table 2). It was observed that there was increase in capsular thickness on 20th day. Gradual reduction in capsular thickness, subcapsular edema and vascular congestion was observed on 40th and 60th days of sacrifice. Only group B3 showed complete recovery in capsular thickness, vascular congestion and subcapsular edema as the effect of drug was wearing off but this change persisted in higher dose groups. These findings are not supported by any previously available research work on testes of experimental animals. Watanabe et al. [12] reported that RBV might have led to arterial and venous obstruction that resulted in vascular congestion and edema. RBV caused vascular dilatation and congestion in capsular vessels. There may be leakage of fluid from these vessels that resulted in capsular and subcapsular edema. It caused increased capsular thickness, that slowly settled with the passage of time in low dose group B3 due to diminished toxic effects of this drug [13].
Regarding subcapsular edema, a prior study highlighted that RBV caused transient pulmonary edema that cured when the drug was abandoned [14]. Pulmonary edema actually develops because of high capillary permeability due to damage of small vessels like capillaries of alveolar septas exposed to medications [15]. RBV can induce injuries of vessels [16], so edema may be a result of injury to vascular endothelium primarily or damage to alveolar epithelium (with secondary injury to small vessels). This can cause oozing of fluid and proteins into interstitial spaces and into the alveoli [15]. Edema is denoted by increased fluid in the interstitial spaces. The opposing effects of hydrostatic pressure of vessels and colloidal osmotic pressure are the main factors that cause movement of fluid between vascular and subcapsular spaces. In normal condition, the fluid release into the subcapsular space from arteriolar end of capillaries is almost stabilized by entrance of fluid at the venular end. When there is increase in capillary pressure or decrease in colloidal osmotic pressure, it can result in more interstitial fluid. Locally hydrostatic pressure may increase due to reduced venous outpouring [13]. RBV might have caused arterial occlusion or venous outflow obstruction according to Watanabe et al. [12]. Therefore, it may have caused the above-mentioned pathology. Hirai et al. [17] reported that testes need plenty of blood flow for spermatogenesis and testosterone production. Blood flow disturbance to the testes, either in the form of venous or arterial occlusion, both result in spermatogenesis problems due to oxidative stress. The venous blockage more commonly disturbs the organs with single venous outflow such as testis and ovary [13].
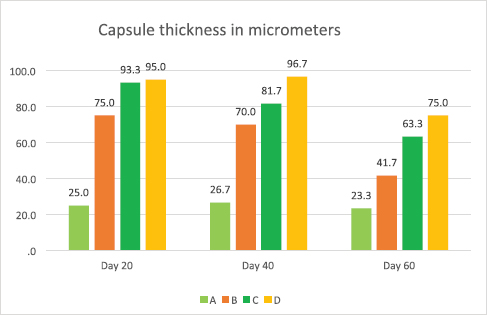
Figure 4. Bar graph showing a comparison of mean capsular thickness between study groups at different times of sacrifice.
Subcapsular edema might be due to change in capillary permeability of the tissue. Narayana et al. [7] noted that whenever seminiferous tubular epithelium deteriorated due to RBV, there was fall in testosterone levels that caused increase in Luteinizing hormone from anterior pituitary. The degeneration of the germ cell population of the testis is associated with changes in the pituitary histology and increase in the gonadotrophin component of the gland. Atallah et al. [18] reported that decreased levels of testosterone may cause increased capillary permeability resulting in blood-brain barrier leakage and in this way, it can affect the testicular microcirculation. Low levels of testosterone might have caused increased capillary permeability and subcapsular edema.
Conclusion
RBV, in addition to its histological effects on testicular seminiferous tubules and its interstitium, exerts toxic effects on the testicular capsule thickness with dose and time-related recovery after discontinuation of treatment.
Limitations of the study
The only limitation in the study was the duration of experiment that was 60 days and high dose treated groups were observed only for that much period. These groups must be studied for longer period of time till the testicular capsule showed full recovery.
Acknowledgements
This work was supported by Department of Anatomy, Postgraduate Medical institute, LHR.
The authors would like to express their sincere thanks to Mrs. Bushra Munir for her assistance in the laboratory work at UHS and Mr. Shahid for taking care of the experimental animals in the research laboratory of PGMI. The authors wish to thank Miss Maham Fatima and Imran Ali Sheikh who assisted in the proof reading and content reviewing of the manuscript.
Conflict of interest
None to declare.
Grant support & financial disclosure
This is self-supported study.
Ethical approval
The protocol of this research was approved by the Institutional Ethical Review Board of University of Health Sciences, Lahore (Letter No. UHS/Education/126-10/91521-06-2019).
Author’s contribution
AB: Substantial contributions to conception and design, Analysis and interpretation of data, drafting the article
MF: Acquisition of data and drafting the article.
FF: Revising the manuscript critically for important intellectual content.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Author details
Alvia Batool1, Maryam Fatima1, Fozia Farzana2
- Department of Anatomy, Fatima Memorial Hospital College of Medicine & Dentistry, Lahore, Pakistan.
- Anatomy Department, Continental Medical College, Lahore, Pakistan.
References
- Autifi MAH, Salem EAA, Abdel Hady EAR, Younis AM. Effect of ribavirin on the testes of adult albino rats (light microscopic study). Nat Sci. 2017;15(11):69–77.
- Hepatitis. Encyclopaedia Britannica, Inc. 2020. [cited 2020 Nov] Available from: https://www.britannica.com/science/hepatitis
- Acosta EP, Flexner C. Antimicrobial agents: antiviral agents (Non retroviral). In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's. The pharmacological basis of therapeutics. 12th ed. New York, NY: Mcgraw Hill Companies; 2011. pp 1613–5.
- Katzung BG, Trevor AJ. Antiviarl agents. In: Basic & clinical pharmacology. 13th ed. Delhi, India: McGraw Hill Education; 2015. pp 859–61.
- Trevor AJ, Katzung BG, Kruidering-Hall M. Antiviarl chemotherapy and prophylaxis. In: Katzung & trevor’s pharmacology. 11th ed. New York, NY: McGraw Hill Education; 2015. pp 408–9.
- Carrillo-Bustamante P, Nguyen THT, Oestereich L, Günther S, Guedj J, Graw F. Determining ribavirin’s mechanism of action against Lassa virus infection. Sci Rep. 2017;7(11693):1–12. https://doi.org/10.1038/s41598-017-10198-0
- Narayana K, D’Souza UJA, Narayan P, Kumar G. The antiviral drug ribavirin reversibly affects the reproductive parameters in male Wistar rat. Folia Morphol. 2005;64(2):6571.
- D’Souza UJ, Narayana K. Mechanism of cytotoxicity of ribavirin in the rat bone marrow and testis. Indian J Physiol Pharmacol. 2002;46(4):468–74.
- Tatar A, Ozkurt Z, Kiki I. Genotoxic effect of ribavirin in patients with Crimean-Congo hemorrhagic fever. Jpn J Infect Dis. 2005;58(5):313–5.
- Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY, Maddrey WC. The ribavirin pregnancy registry: an interim analysis of potential teratogenicity at the mid-point of enrolment. Drug Saf. 2017;40(12):1205–18. https://doi.org/10.1007/s40264-017-0566-6
- Moustafa HM, Aly AWF, Eid KA, Soliman MMAM, Alsied ARA. Effect of pegylated interferon and ribavirin used for treatment of chronic hepatitis C patients on semen parameters. Al-Azhar Assiut Med J. 2012;10(1):76–94.
- Watanabe M, Ogasawara S, Takahashi A, Takada J, Tanaka Y, Okuwaki Y, et al. Branch retinal artery occlusion and central retinal vein occlusion associated with pegylated interferon plus ribavirin combination therapy for chronic hepatitis C. Cutan Ocul Toxicol. 2011;31(3):253–7. https://doi.org/10.3109/15569527.2011.641197
- Mitchell RN. Hemodynamic disorders, thromboembolic disease and shock. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robins and cotran pathological basis of disease. 9th ed. Philadelphia, PA: Saunders, Elsevier Inc; 2015. 125–6 pp.
- Virazole. Rx List. 2020. [cited 2020 Dec] Available from: https://www.rxlist.com/virazole-drug.htm#description
- Husain AN. The lung. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robins and cotran pathological basis of disease. 9th ed. Philadelphia, PA: Saunders, Elsevier Inc; 2015. pp 671–3.
- Copegus. Roche Laboratories Inc. 2020. [cited 2020 December] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021511s012lbl.pdf
- Hirai S, Hatayama N, Naito M, Nagahori K, Kawata S, Hayashi S, et al. Pathological effect of arterial ischaemia and venous congestion on rat testes. Sci Rep. 2017;7(5422):1–9. https://doi.org/10.1038/s41598-017-05880-2
- Atallah A, Mhaouty-Kodja S, Grange-Messent V. Chronic depletion of gonadal testosterone leads to blood-brain barrier dysfunction and inflammation in male mice. J Cereb Blood Flow Metab. 2017;37(9):3161–75. https://doi.org/10.1177/0271678X16683961
Keywords: Testes, Rat, Cytotoxicity, Capsule, Thickness, Ribavirin.
Publication History
Received: November 19, 2020
Revised: May 08, 2021
Accepted: May 29, 2021
Published: June 30, 2021
Authors
Alvia Batool
Department of Anatomy, Fatima Memorial Hospital College of Medicine & Dentistry, Lahore - Pakistan.
Maryam Fatima
Department of Anatomy, Fatima Memorial Hospital College of Medicine & Dentistry, Lahore - Pakistan.
Fozia Farzana
Anatomy Department, Continental Medical College, Lahore - Pakistan.
