Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 38(2):57-63
REVIEW ARTICLE
Agonists and antagonists of the orexinergic system: therapeutic molecules of the future - a narrative review
Zunaira Akram1, Sarah Ghafoor2* 
Received: 10 January 2022 Revised date: 13 April 2022 Accepted: 02 June 2022
Correspondence to: Sarah Ghafoor
*Assistant Professor, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan.
Email: sarahghafoor@uhs.edu.pk
Full list of author information is available at the end of the article.
ABSTRACT
The orexinergic system involves orexin (OX) neurons, OX peptides, and OX receptors. OX neurons are located throughout the central nervous tissue (including many nuclei) and the peripheral nervous tissue and organs. These neurons are critically involved in the sleep-wake transition, cardiorespiratory, and autonomic regulations. OX antagonists include selective OX type 1 receptor (OX1R) antagonists, selective OX type 2 receptor (OX2R) antagonists, and dual OX1/2R antagonists. Similarly, OX agonists include dual OX1/2R agonists and selective OX1R or OX2R agonists. Recent understandings of the therapeutic mechanism of OX have led to possible therapeutic options in diseases such as insomnia, narcolepsy, psychiatric disorders, and neurodegenerative diseases. This review focuses on the therapeutic roles of OXs as agonists or antagonists in animal models and human patients, which can lead to possible avenues related to their application in health and disease.
Keywords:
Orexin, agonists, antagonists, narcolepsy, insomnia, Alzheimer’s disease.
Introduction
Orexin (OX) is a neuropeptide that is also known as hypocretin. It is produced in the form of pre-proorexin which then undergoes proteolytic processing to form two peptides: orexin-A (OX-A) and orexin-B (OX-B). OX peptides are then packed in vesicles and released synaptically; however, little is known about their further kinetics.1,2 OX-A and OX-B have 33 and 28 amino acids, respectively. These peptides are produced by clusters of neurons in the hypothalamus, extending mediolaterally from the dorsomedial hypothalamic nucleus to lateral hypothalamus through prefornical area. OX signals are mediated through two heterotrimeric G-protein-coupled receptors: OX type-1 receptor (OX1R) and OX type-2 receptor (OX2R). Receptor activation of OX neurons involves postsynaptic depolarization through Ca2+ influx, K+ channel inhibition, and Na+/Ca2+ exchanger with activation of nonselective cation channels.3,4 These OX neurons, peptides, and receptors form the orexinergic system of the human body. The physiological functions of OX are summarized in Table 1.
OX afferent neurons receive input from the amygdala, insular cortex, and nucleus accumbens. OX efferent neurons innervate the nuclei for signaling, such as locus coeruleus for nonadrenergic, tuberomamillary for histaminergic, raphe nuclei for serotonergic, ventral tegmental area for dopaminergic and basal forebrain for cholinergic and noncholinergic signaling (Figure 1).5 Any increase or decrease in the levels of OX leads to atrocious effects of various pathologies such as narcolepsy, insomnia, psychiatric disorders, and neurodegenerative diseases such as Alzheimer’s disease. OX antagonists include selective OX1R antagonists, selective OX2R antagonists, and dual OX1/2R antagonists. Similarly, OX agonists include dual OX1/2R agonists and selective OX1R or OX2R agonists. Mouse models, such as CAG / OX and OX flippase, and knock-in mice model, have been used to demonstrate the physiological malfunctioning of OX but providing an agonists or antagonists of OX ameliorates such metabolically and physiological dysfunctioning (Figure 2).6-8
The aim of the narrative review is to provide clinicians with updated information regarding various roles and therapeutic possibilities of agonists and antagonists of the OX system. Articles of the last 10 years were searched using search engines, such as PubMed and Google Scholar. Medical subject heading terms were used as keywords in various combinations, such as orexinergic system, OX antagonists, OX agonists, narcolepsy, insomnia, Alzheimer’s disease, diabetes mellitus, cancer, cocaine, and alcohol intoxication. Research publications that were available in the English language only were included. The scope of the search was focused to the orexinergic system and its therapeutic role in the form of OX agonists and antagonists in various diseases. Manuscripts in languages other than English and those that could not be retrieved as full-text articles were excluded. Henceforth, the role of OX agonists and antagonists in sleep-related disorders, as well as in other pathologies, has been discussed in this review.
Table 1. Physiological functions of OXs.
| Serial no. | Systems | Functions |
|---|---|---|
| 1. | Hypothalamic regulation | Sleep/wake transition. |
| Feeding behavior. | ||
| Energy homeostasis. | ||
| Thermoregulation. | ||
| 2. | Gastrointestinal regulation | Insulin secretion. |
| Intestinal motility. | ||
| Gastric acid secretion. | ||
| Duodenal bicarbonate secretion. | ||
| Stimulation of protein and fluid in stomach. | ||
| Inhibits lipolysis. | ||
| 3. | Cardiorespiratory regulation | Increases blood pressure. |
| Increases heart rate. | ||
| Sympathetic nerve activity. | ||
| Regulates amplitude and frequency of respiration. | ||
| 4. | Mechanical regulation | Locomotor activity (spontaneous physical activity). |
| 5. | Other regulatory functions | Drug-seeking behavior (reward and reinforcement). |
| Cognitive functions. | ||
| Release of epinephrine and glucocorticoid. | ||
| Antitumor properties (proapoptotic effects). | ||
| Sensory modulation. |
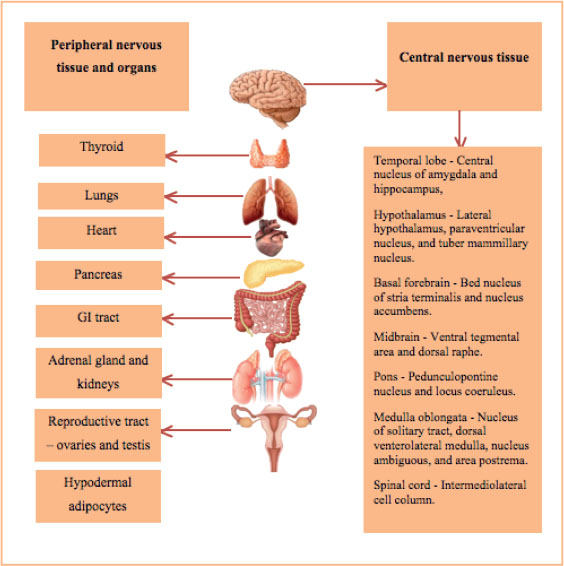
Figure 1. Presence of the orexinergic system in the human body. Left panel shows the expression/identification of the orexinergic system in peripheral tissues and organs. Right panel shows the expression/identification of orexinergic system in central nervous tissue.
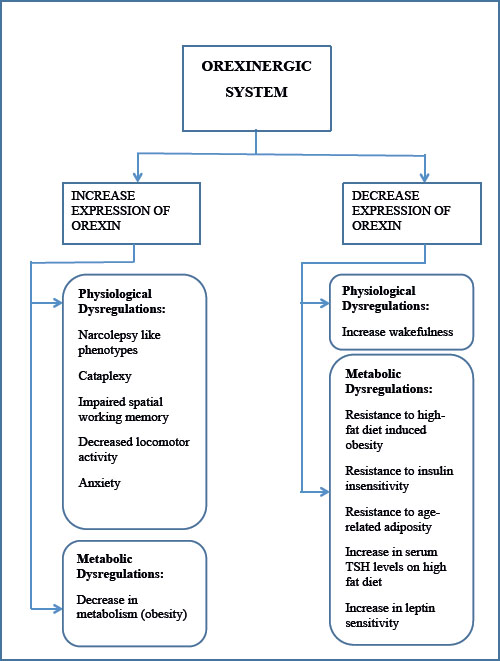
Figure 2. Increase and decrease in the expressions of the orexinergic system in a mice model. Left panel shows the physiological and metabolic dysregulations in a knockout mice model. Right panel shows the physiological and metabolic dysregulations by ectopic overexpression of the Orexinergic system in a mice model. TSH: Thyroid Stimulating Hormone.
OX Antagonists and Sleep-Related Disorders
Sleep is critical for normal physiological functioning of the human body. Sleep deprivation plays a pivotal role in pathophysiology of various diseases, such as insomnia and diabetes mellitus, and psychiatric disorders, such as anxiety and seizures, and neurodegenerative diseases, such as Alzheimer’s disease. The orexinergic system has been identified as the key regulator of the sleep/wake cycle and maintains the wakefulness state of mind. OX neuropeptides regulates the sleep/wake transition by casting its noradrenergic effect on locus coeruleus, histaminergic effect on tuberomammillary nucleus, serotonergic effect on dorsal raphe nucleus, and cholinergic effect on laterodorsal tegmental area/pedunculopontine tegmental area.
Previous medications for the treatment of insomnia include benzodiazepines, Z-drugs (which are receptor modulators of nonbenzodiazepine gamma-aminobutyric acid type A, and melatonin receptor agonists. Overt side effects of these medications include drug dependence, intolerance to drugs, daytime sleepiness, and cognitive impairment. Some of these hypnotic medications also manifest breathing disorders during sleep in patients with chronic obstructive respiratory disease.9 Current prescription for the treatment of insomnia includes OX receptor antagonists. Among the list of accepted OX antagonists, Suvorexant and Lemborexant are available in clinical practice and Daridorexant and Seltorexant are under clinical development.10
Clinical trial with Suvorexant has shown an early onset of sleep in nonelderly and elderly patients of insomnia.11 As compared to other sleep medications, Suvorexant has no overt respiratory depressant effect in patients with chronic obstructive respiratory disease.12 In patients with Alzheimer’s disease, clinical trial with oral Suvorexant has shown to improve sleep on the primary end point of the total sleep time and secondary end point of the wake-after-persistent-sleep onset. It has also shown to attenuate psychological symptoms of dementia in Alzheimer’s patients. Somnolence, a state of feeling drowsy, was the only adverse side effect reported in Alzheimer’s patients.13
OX neurons have been identified in brain areas critical for regulation of stress and anxiety-related responses, which include central nucleus of the amygdala, bed nucleus of stria terminalis, medial prefrontal cortex, and hippocampus. In these regions, OX regulates many neuroendocrine, metabolic, and sympathetic functions.14 Clinical trial with Suvorexant has shown to improve sleep duration in patients with anxiety. It has also shown to decrease the period of anxiety and depression, and hypothalamic-pituitary-adrenal axis markers, such as fasting plasma cortisol level and white blood cell count, and also casts some effect on sympathetic markers such as fasting plasma noradrenaline level and pulse rate.15
Apart from central nervous tissue regulations, OX receptors also regulate peripheral tissue and organs. While stabilizing the sleep/wake transition, OX receptors also modify the hepatic metabolism and increase glucose consumption in the skeletal muscles by acting through sympathetic and parasympathetic nervous systems.16 Insomnia and type 2 diabetes mellitus are the two most commonly reported symptoms of ageing as they exacerbate each other. An improvement in sleep quality has shown to improve glycemic control. In a diabetic mice model, Suvorexant has shown to manifest an increase in nonrapid eye movement sleep and also improved hepatic glucose metabolism. Similarly, in type 2 diabetic patient with insomnia, Suvorexant improved sleep efficiency and glycemic control with partial effect on sympathetic tone.17
Accumulation of neurotoxic waste in the central nervous tissue leads to neuroinflammation, which has shown to increase the progression of Alzheimer’s disease. The dysfunctional OX system exhibits a sleep disorder called the irregular sleep-wake rhythm disorder (ISWRD) in patients with Alzheimer’s disease. Hence, any change in the levels of OX fastens the pathology of Alzheimer’s disease either by disturbing sleep, aggravating mitochondrial impairment, or through genetic vulnerability.18 Lemborexant is another dual OX receptor antagonist and it has been recently approved by the U.S. Food and Drug Administration (FDA) for the treatment of insomnia. Clinical trial with oral doses of lemborexant has shown to improve the 24-hour circadian rhythm and nocturnal sleep variables in patients with ISWRD of Alzheimer’s disease. Lemborexant also has shown improved tolerability among Alzheimer’s patients.19
Almorexant is another dual receptor antagonist that has shown to maintain sleep with less cognitive impairment through selective defacilitation of the wake-promoting system rather than complete inhibition of neural activity. Almorexant also decreases the sleep onset time and maintains good sleep without its withdrawal effects on the next-day performance.20
OX Agonists in Sleep-Related Disorders
An orthosteric, nonpeptide OX2R selective agonists is YNT-185. It ameliorates narcolepsy symptoms and excessive sleepiness due to other causes, such as idiopathic hypersomnia, sleepiness accompanying depression, and jet lag or shift work, and increases the wakefulness state in a dose-dependent manner. Intraperitoneal and intracerebroventricular administration of YNT-185 suppresses cataplexy-like episodes more in OX knockout mice than OX receptor-deficient mice.21 Obesity is one of the metabolic effects of narcolepsy. The narcolepsy rodent mouse model also tends to develop late onset obesity. Intraperitoneal administration of YNT-185 showed a significant decrease in body weight of OX knockout mice as compared to OX receptor-deficient mice. Upon termination of YNT-185, body weight was gradually regained, suggesting that its effects were found to be reversible. However, it has been considered not suitable for further clinical development due to its limited efficacy and central nervous system (CNS) bioavailability. A very high concentration of this nonpeptide agonist is required for desired pharmacological effects.22
TAK-925 is another selective OX2R agonist. It has been shown that TAK-925 possesses the desired therapeutic potential for the treatment of hypersomnia and associated diseases, such as narcolepsy type-1. In OX knockout mice, it has shown to induce neural activation and arousal. It also increases wakefulness in Wister mice and decreases both nonrapid eye movement and rapid eye movement sleep duration. TAK-925 is under investigation as a potential source of treatment option for patients with sleep disorders and as an adjunct OX replacement therapy.23 RTOXA-43 (compound 40) has been identified as a dual OX receptor agonist. Through electroencephalogram/electromyography recordings in 12-month-old mice, compound 40 has shown to increase wake time by increasing the duration of episode, decrease the nonrapid eye movement and rapid eye movement sleep, and improve the sleep/wake transition. This research study also proposed a dual OX receptor agonist as a possible treatment option for diseases such as narcolepsy.24
OX Agonists and Antagonists in Other Pathologies
OX neurons and receptors are widespread in brain areas such as paraventricular nucleus, rostral ventrolateral medulla, and nucleus ambiguous, area postrema, nucleus tractus solitaries, and spinal cord sympathetic preganglionic neurons which controls blood pressure, sympathetic nerve activity, and cardiovascular responses. The oral administration of Almorexant produces a significant decrease in blood pressure and heart rate in hypertensive mice and causes no significant effect on normotensive mice, indicating the role of OX in pathophysiology of hypertension. Hence, it is suggested to prescribe an OX antagonist drug for maintaining the blood pressure and heart rate in hypertensive patients.25 In a rat model study, Almorexant has also shown to reduce dopamine-induced cocaine responses and cocaine self-administration behavior.26 Being a calcium pathway dual OX receptor antagonist, Almorexant, also behaves as a full apoptotic pathway agonist. Intraperitoneal injection of almorexant in a xenografted mouse model of pancreatic ductal carcinoma manifested a 50% decrease in tumor size and antitumoral properties such as activation of caspases.27
Cocaine intoxication causes an increase in motor impulsivity and transient impulsive behavior through phasic dopamine signaling. Suvorexant has shown to attenuate cocaine-induced impulsive behavior, its motivational properties,28 and improves rapid eye movement sleep (as shown in the rat model exposed to chronic alcohol consumption).29 Another antagonist SB-334867 also decreases the motivation for cocaine and alcohol self-administration and attenuated naloxone-induced morphine withdrawal symptoms.30
Previous literature and research works have been exploited on OX antagonists, but little research is available for OX agonists. Many antagonists have also been approved by the FDA for the treatment of insomnia, and OX agonists are still under clinical development. The data available on partial agonists can be suggested as a good starting point for further research on more potent OX agonists. To evaluate OX receptor agonists, techniques such as stapled or modified alpha-isoaminobutyric acid (Aib) peptides reported by Karhu et al. 31 can be used to bring out the potential pharmacological effects of OX. These techniques have shown to enhance the receptor binding and decrease the protease cleavage rates.
Possible Routes for OX Supplementation
The most common therapeutic approaches in the treatment of various pathologies include oral, intranasal, and intravenous supplementations. The delivery of therapeutics to the CNS is limited by the blood-brain barrier (BBB). In this regard, intranasal delivery is the optimal method as a noninvasive bypass of the BBB, targeting the specific brain and spinal cord regions through olfactory and trigeminal neural pathways. Intranasal administration of OX-A has shown 10-fold lower concentrations in blood and kidneys as compared to other pathways. Other routes for OX administration are being explored which include gene therapy and cell transplantation.22
Future Prospects and Conclusion
Dysregulation in OX functions leads to various inflammatory, systemic, and neurodegenerative disorders. Targeting orexinergic system in the body can prove to be a promising strategy for the treatment of such disorders. Many antagonists limit the application of endogenous agonists in normal physiological process, so further future clinical trials are required to formulate products with better physiochemical properties. Appropriate identification and utilization of these agonists and antagonists can contribute to reality in the field of medicine. It can be speculated that filling up the gaps in therapeutic impediments can help the clinicians in attenuating the disease condition.
Acknowledgement
The authors are thankful to the University of Health Sciences Lahore and the Higher Education Commission for providing access to e-library through which indexed research journals were accessed free of cost.
List of Abbreviations
| Aib | Alpha-isoaminobutyric acid |
| BBB | Blood-brain barrier |
| Ca2+ | Calcium |
| CNS | Central nervous system |
| FDA | Food and drug administration |
| ISWRD | Irregular sleep-wake rhythm disorder |
| K+ | Potassium |
| Na+ | Sodium |
| OX | Orexin |
| OX-A | Orexin-A |
| OX-B | Orexin-B |
| OX2R | Orexin type 2 receptor |
| OX1R | Orexin type 1 receptor |
Conflict of interest
None to declare.
Grant support and financial disclosure
The manuscript is extracted from the M. Phil project of the principal author. The project was financially supported by the University of Health Sciences, Lahore, Pakistan.
Ethical approval
Not required.
Authors’ contributions
ZA: Acquisition of data, data analysis, and manuscript writing.
SG: Conception and design of study, important intellectual input, and drafting of manuscript.
Both authors approved the final version of the manuscript to be published.
Authors’ Details
Zunaira Akram1, Sarah Ghafoor2
- M. Phil Student, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
- Assistant Professor, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
References
- Boss C, Roch C. Orexin research: patent news from 2016. Expert Opin Ther Pat. 2017;27(10):1123–33. https://doi.org/10.1080/13543776.2017.1344221
- Liu L, Wang Q, Liu A, Lan X, Huang Y, Zhao Z, et al. Physiological implications of Orexins/Hypocretins on energy metabolism and adipose tissue development. ACS Omega. 2020;5(1):547–55. https://doi.org/10.1021/acsomega.9b03106
- Kukkonen JP. Orexin/Hypocretin signaling. Curr Top Behav Neurosci. 2017;33:17–50. https://doi.org/10.1007/7854_2016_49
- Wang C, Wang Q, Ji B, Pan Y, Xu C, Cheng B, et al. The Orexin/receptor system: Molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. https://doi.org/10.3389/fnmol.2018.00220
- Scammell TE, Winrow CJ. Orexin receptors: Pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51(1):243–66. https://doi.org/10.1146/annurev-pharmtox-010510-100528
- Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, et al. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9(1):64–76. https://doi.org/10.1016/j.cmet.2008.10.010
- Chowdhury S, Hung CJ, Izawa S, Inutsuka A, Kawamura M, Kawashima T, et al. Dissociating orexin-dependent and -independent functions of orexin neurons using novel Orexin-Flp knock-in mice. Elife. 2019;8:e44927. https://doi.org/10.7554/eLife.44927
- Mori T, Uzawa N, Masukawa D, Hirayama S, Iwase Y, Hokazono M, et al. Enhancement of the rewarding effects of 3,4-methylenedioxymethamphetamine in orexin knockout mice. Behav Brain Res. 2021;396:112802. https://doi.org/10.1016/j.bbr.2020.112802
- Lieberman JA. Update on the safety considerations in the management of insomnia with hypnotics: incorporating modified-release formulations into primary care. Prim Care Companion J Clin Psychiatry. 2007;9(1):25–31. https://doi.org/10.4088/pcc.v09n0105
- Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol. 2020;16(11):1063–78. https://doi.org/10.1080/17425255.2020.1817380
- Herring WJ, Connor KM, Ivgy-May N, Snyder E, Liu K, Snavely DB, et al. Suvorexant in patients with Insomnia: results from two 3-month randomized controlled clinical trials. Biol Psychiatry. 2016;79(2):136–48. https://doi.org/10.1016/j.biopsych.2014.10.003
- Sun H, Palcza J, Rosenberg R, Kryger M, Siringhaus T, Rowe J, et al. Effects of suvorexant, an orexin receptor antagonist, on breathing during sleep in patients with chronic obstructive pulmonary disease. Respir Med. 2015;109(3):416–26. https://doi.org/10.1016/j.rmed.2014.12.010
- Herring WJ, Ceesay P, Snyder E, Bliwise D, Budd K, Hutzelmann J, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16(3):541–51. https://doi.org/10.1002/alz.12035
- Sargin D. The role of the orexin system in stress response. Neuropharmacology. 2019;154:68–78. https://doi.org/10.1016/j.neuropharm.2018.09.034
- Nakamura M, Nagamine T. Neuroendocrine, autonomic, and metabolic responses to an Orexin antagonist, Suvorexant, in psychiatric patients with insomnia. Innov Clin Neurosci. 2017;14(3-4):30–7.
- Tsuneki H, Kon K, Ito H, Yamazaki M, Takahara S, Toyooka N, et al. Timed inhibition of Orexin system by Suvorexant improved sleep and glucose metabolism in type 2 diabetic db/db mice. Endocrinology. 2016;157(11):4146–57. https://doi.org/10.1210/en.2016-1404
- Toi N, Inaba M, Kurajoh M, Morioka T, Hayashi N, Hirota T, et al. Improvement of glycemic control by treatment for insomnia with suvorexant in type 2 diabetes mellitus. J Clin Transl Endocrinol. 2019;15:37–44. https://doi.org/10.1016/j.jcte.2018.12.006
- Moline M, Thein S, Bsharat M, Rabbee N, Kemethofer-Waliczky M, Filippov G, et al. Safety and efficacy of lemborexant in patients with irregular sleep-wake rhythm disorder and Alzheimer’s disease dementia: results from a phase 2 randomized clinical trial. J Prev Alzheimers Dis. 2021;8(1):7–18. https://doi.org/10.14283/jpad.2020.69
- Scott LJ. Lemborexant: first approval. Drugs. 2020;80(4):425–32. https://doi.org/10.1007/s40265-020-01276-1
- Black J, Pillar G, Hedner J, Polo O, Berkani O, Mangialaio S, et al. Efficacy and safety of almorexant in adult chronic insomnia: a randomized placebo-controlled trial with an active reference. Sleep Med. 2017;36:86–94. https://doi.org/10.1016/j.sleep.2017.05.009
- Irukayama-Tomobe Y, Ogawa Y, Tominaga H, Ishikawa Y, Hosokawa N, Ambai S, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci U S A. 2017;114(22):5731–6. https://doi.org/10.1073/pnas.1700499114
- Nepovimova E, Janockova J, Misik J, Kubik S, Stuchlik A, Vales K, et al. Orexin supplementation in narcolepsy treatment: a review. Med Res Rev. 2019;39(3):961–75. https://doi.org/10.1002/med.21550
- Yukitake H, Fujimoto T, Ishikawa T, Suzuki A, Shimizu Y, Rikimaru K, et al. TAK-925, an orexin 2 receptor-selective agonist, shows robust wake-promoting effects in mice. Pharmacol Biochem Behav. 2019;187:172794. https://doi.org/10.1016/j.pbb.2019.172794
- Zhang D, Perrey DA, Decker AM, Langston TL, Mavanji V, Harris DL, et al. Discovery of arylsulfonamides as dual orexin receptor agonists. J Med Chem. 2021;64(12):8806–25. https://doi.org/10.1021/acs.jmedchem.1c00841
- Imperatore R, Palomba L, Cristino L. Role of Orexin-A in hypertension and obesity. Curr Hypertens Rep. 2017;19(4):34. https://doi.org/10.1007/s11906-017-0729-y
- Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6(1):138–46. https://doi.org/10.1021/cn500246j
- Dayot S, Speisky D, Couvelard A, Bourgoin P, Gratio V, Cros J, et al. In vitro, in vivo and ex vivo demonstration of the antitumoral role of hypocretin-1/orexin-A and almorexant in pancreatic ductal adenocarcinoma. Oncotarget. 2018;9(6):6952–67. https://doi.org/10.18632/oncotarget.24084
- Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, et al. Effects of Suvorexant, a dual Orexin/Hypocretin receptor antagonist, on impulsive behavior associated with cocaine. Neuropsychopharmacology. 2018;43(5):1001–9. https://doi.org/10.1038/npp.2017.158
- Sanchez-Alavez M, Benedict J, Wills DN, Ehlers CL. Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep. 2019;42(4):zsz020. https://doi.org/10.1093/sleep/zsz020
- Han Y, Yuan K, Zheng Y, Lu L. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull. 2020;36(4):432–48. https://doi.org/10.1007/s12264-019-00447-9
- Karhu L, Weisell J, Turunen PM, Leino TO, Pätsi H, Xhaard H, et al. Stapled truncated orexin peptides as orexin receptor agonists. Peptides. 2018;102:54–60. https://doi.org/10.1016/j.peptides.2018.02.004
Keywords: Orexin, agonists, antagonists, narcolepsy, insomnia, Alzheimer’s disease
Publication History
Received: January 10, 2022
Revised: April 13, 2022
Accepted: June 02, 2022
Published: June 20, 2022
Authors
Zunaira Akram
M. Phil Student, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
Sarah Ghafoor
Assistant Professor, Department of Oral Biology, University of Health Sciences, Lahore, Pakistan
