Biomedica - Official Journal of University of Health Sciences, Lahore, Pakistan
Volume 37(2):76-85
REVIEW ARTICLE
Novel targets and entities inducing cellular apoptosis and anti-angiogenic activity in retinoblastoma management
Zirwa Abdul Rauf1, Muhammad Hamza Zahid1, Feng Guo1, Zaigui Wang1*
Received: 05 January 2021 Revised date: 31 March 2021 Accepted: 01 June 2021
Correspondence to: Zaigui Wang
*College of Life Science, Anhui Agricultural University, Hefei, China.
Email: wangzaigui2013@163.com
Full list of author information is available at the end of the article.
ABSTRACT
Retinoblastoma (Rb) represents a primary pediatric cancer, which if left untreated can invade to the nervous system that primarily occurs due to loss of the RB1 gene. Several clinically available therapies are used for the management of risk factors associated with Rb including chemotherapy, brachytherapy, external beam radiotherapy etc. However each treatment has its own side effects. To meet with the best approaches in order to minimize these side effects novel targeted therapies have been developed that inhibits tumor in an angiogenic-dependent manner. This review provides the insights about some targets and the pharmaceuticals with their possible mechanism of action that targets angiogenesis and induces apoptosis. The targets include activation of p53 via controlling mouse double minute homolog 2, survivin, and thrombospondin-1. Entities described in this review include 5-aminoimidazole-4-carboxamide ribonucleotide, niclosamide, bevacizumab, aflibercept, genistein and quercetin and their potential in treating Rb. Also, the signaling pathways that are affected in response to these drugs like activated protein kinase pathway, Wnt/β-catenin pathway, vascular endothelial growth factor and its receptors has also been discussed.
Keywords:
Retinoblastoma, p53 activation, Angiogenesis inhibitors, Signaling pathways.
Introduction
Retinoblastoma (Rb)- origin and indication
Rb is an intermittent intraocular invasive cancer which is capable of severely metastasizing central nervous system via invading sclera and post laminar optic nerve [1,2]. Rb has higher prevalence in infants and is ranked at the topmost, with virtual frequency of 3% among existing pediatric malignancies [3]. In 2009, the expected death rate due to this evil was about 4,000 per year across the globe [4]. Rb is an autosomal disorder that predominantly ensue as a consequence of mutations in RB1 gene or amplification of MYCN oncogenes [5]. RB1, a gene that is placed on chromosome no. 13, possesses the potential to prevent the dissemination of tumor [6]. Rb could either be inherited or acquired. Hereditary Rb involves biallelic inactivation of RB1 gene (mutation transmitted through defective allele in germ cells and the succeeding loss of the second allele somatically) and might perhaps lead to unilateral or bilateral retinal cancers. Whereas, purely somatic variation is the reason for sporadic Rb which only leads to unilateral tumors whether due to RB1 gene inactivation (biallelic inactivation) or due to the MYCN oncogenes [7,8]. Adding to these, several other hereditary mutations that are either DNA-linked or linked to other factors and formerly found to have regulatory roles in succession of phases happening in cell division cycle or controlled cellular death and/or angiogenesis also plays a basic role in the development and dissemination of tumors [9,10]. For comprehensive review regarding heterogeneity in Rb and the involvement of RB1 in cell cycle and tumor progression see (heterogeneity in Rb: a tale of molecules and models) [11].
According to a research study undertaken in South Western China in 2016, this tumor is indicated by conditions like leukocoria which is then followed by proptosis, lacrimation, ocular pain, strabismus etc. [12].
Therapeutic approaches for treating Rb depends upon the diagnosis stage and invasiveness. Enucleation, chemotherapy, radiotherapy (external beam radiotherapy mostly used) and adjunctive therapy (cryotherapy/laser photocoagulation) are generally in practice [13]. However, the use of these treatment modalities possibly will lead to the vision loss or impairment (relapse) as well as the development of secondary tumors (as a result of radiation therapy) [14]. Therefore, alternative therapies must be needed to repress tumorigenesis [15].
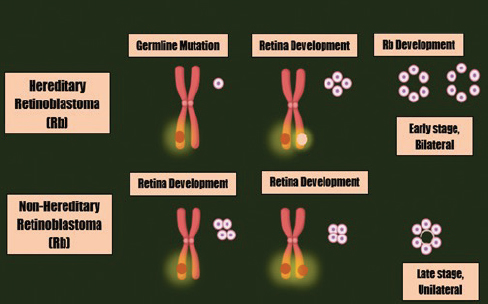
Figure 1. The mutations leading to Rb development.
Novel therapeutic targets for Rb
miRNA-31 and miRNA-200a
MicroRNAs are RNA transcripts that are relatively small and are not expressed as protein; however, they have the potential to facilitate and control the expression of a protein from specific genes, and their divergent expression often leads to ailment. MiRNA-31 has presented its anti-tumor activity in many cancer models [16].
MiRNA-200a has its place in the family of miRNAs that substantially regulates cancer progression, entry or exit of a cell in quiescent state and differentiation of pluripotent neuronal cells by regulating numerous oncogenes [17]. MiR-200a pursues prominent concern because it down-regulates Wnt/β-catenin pathway as Wnt ligand in stimulated form aggrandize the growth of population of highly malignant cancerous stem cells of within Rb cell cultures [18].
For the purpose of controlling Rb, anticipating miRNA-31 and/or -200a as a possible therapeutic target is reassuring in order to put a limit on the expansion of this tumor which accordingly decreases the likelihood of metastasis of retinal cancer cells to other ocular structures. Exploring such targets is critical since literature indicates the progression of retinal carcinoma via invading optic nerve as a significant clinical feature with unreceptive prognostic value for the subject. Exploration studies embark on for prevention of Rb via these micro-RNAs signposts that if the genes coding for such functional RNAs (miRNA-31 and miRNA-200a) are down-regulated, then Rb proliferation occurs through selective downregulation of targets linked with prompt cell division [19].
Thus, both of these miRNAs potentially represent novel targets to preclude the metastasis and invasion of Rb as these inhibits cellular exponentiation when their expression is enough [19].
Survivin
Survivin, the smallest member of the family of inhibitors of apoptosis proteins, is a candidate for targeted therapy of cancer. It is a multi-task protein that has been allocated to accomplish varied functions in different cellular organelles including regulation of mitosis and inhibition of apoptosis. The expression level of survivin is virtually negligible in the normal cells but it becomes wrong way up in tumor cells [20-22]. Former investigation gives the evidence for the presence of survivin in Rb patients [23]. The amount of the protein is found to be greater in aqueous humor and serum of them affected individuals with retinal cancer [24]. Numerous approaches have been employed to lessen the expression of survivin to get the improved treatment outcomes. The approach used is reliant on the type of tumor. For instance, experiments have been designed to target survivin using imidazolium-based agent, sepantronium bromide (YM155) to improve healing for Rb patients [25]. Likewise, surviving level in tumor cells can be lowered via silencing the gene expression coding for this protein, at mRNA level by specific small interfering RNAs (siRNA). Handling of Rb through this target could force cancerous cells to become sensitive for chemotherapy and constrains tumor invasion [24].
Thrombospondin-1 (TSP-1)
TSP-1, a protein that is secreted into the extracellular environment has differential expression in different types of tumor cells and is found to exhibit its effect via inhibiting angiogenesis [26]. Although there are controversies about the level of TSP-1 in tumors whether it inhibits tumor via anti-angiogenic activity or promotes tumor growth via neovascularization. Previous literature about TSP-1 indicates that the bone marrow stromal cells-based secretion of TSP-1 improves the survival of retina prospecting the TSP-1 a captivating target for Rb therapy securing the retina as well [27].
p53 activation
The progression of Rb is considered to be cone precursor dependent tumor. The cone features that are obligatory for Rb invasion includes increased expression of the oncogenes such as MYCN and mouse double minute homolog 2 (MDM2) [28]. Based on the retrospective findings, p53 is discovered to be in inactive form in nearly 75% of Rb patients [29]. p53 inactivation occurs due to the accumulation of MDM2 in retinal cancer cells which then restrains p53 mediated apoptosis. Using the inhibitors for MDM2 (Nutlin-3a) supports the concept of targeted therapy in Rb where MDM2 down-regulation leads to the activation of p53 tumor suppressor pathway [30].
Target Molecules Inhibiting Tumor-Angiogenesis and Inducing Apoptosis as Latest Therapeutics for Rb
After the mid-1900s, Folkman et al. [31] revealed angiogenesis as probable cause for tumors. Progression of Rb is discovered to be angiogenesis dependent. Thus, angiogenic inhibitors may suppress the development of retinal carcinoma [32].
5-Aminoimidazole-4-Carboxamide Ribonucleotide (AICAR); an Activator of Activated Protein Kinase (AMPK) Pathway
AICAR, an activator of AMPK, has revealed as an anti-cancerous agent against multiple myeloma cells [33]. Arithmetic figures of the research outcomes indicate the contribution of AMPK in cancer [34]. AMPK appear to react in a number of cell behaviors and lies at the key point of tumor suppressor network [35]. Further investigations in cancer biology have found that the progression and multiplication of Rb cells can be suppressed by pharmacological activation of AMPK [36]. AMPK is a serine/threonine heterotrimeric protein kinase and has three subunits i.e., α, β, and γ so as to make tissue-specific complexes [37,38]. Adenosine Monophosphate (AMP) (an adenosine nucleotide) is a direct activator of AMPK and binds to its γ-domain. Among the three known upstream regulators of AMPK, Liver Kinase B1 (LKB1) is found to have tumor suppressing potency indicating AMPK as a probable target for tumor control [39].
AICAR, after administration, is transformed to Structural analog of AMP (ZMP), an imitator of AMP, and binds to the γ-subunit of AMPK. Advances in research have exhibited that AICAR can be utilized to regress tumor cell proliferation in Rb by S-phase arrest, inducing apoptosis and repressing neovascularization [40]. AICAR activated AMPK leads to the increased expression of p21 which is a cell cycle inhibitory protein. It also partially inhibits mammalian target of rapamycin (mTOR) pathway [36].
Niclosamide: a suppressor of Wnt-signaling pathways
Niclosamide [(5-Chloro-N-2-Chloro-4-Nitrophenyl)-2-hydroxybenzamide], is approved by Food and Drug Administration (FDA) as an anti-helminthic drug against tape worm infections [41].
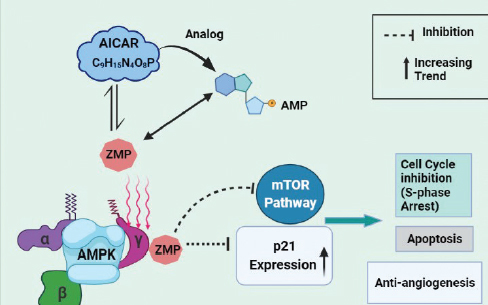
Figure 2. Proposed mechanism of action of AICAR in Rb.
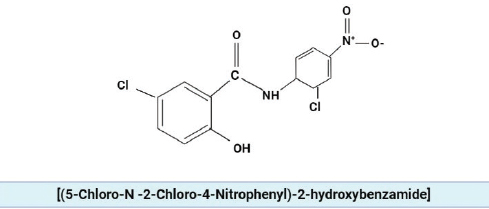
Figure 3. The chemical structure of niclosamide.
High throughput screening has exhibited that niclosamide display great anti-tumor potential as it has been shown to inhibit cellular proliferation in various types of cancers [42,43]. As an anti-cancerous agent, niclosamide inhibits one of three signaling pathways: Wnt-signalling pathways (Wnt/β-catenin pathway) and the suppression of Signal Transducer and Activator of Transcription 3 (STAT3) and NF-ĸB pathway [44].
Recent published literature indicates the potential of niclosamide against Rb demonstrating that it effectively hinders angiogenesis thereby decreasing retinal cancer invasion. The preclinical assessment has shown that niclosamide has had its inhibitory effect only on the Wnt/β-catenin pathway while the STAT3 pathway remains unaffected in Rb upon the administration of niclosamide. The Wnt signaling pathway regulates tumorigenesis, metastasis, cellular differentiation and apoptosis [45]. The canonical Wnt signaling proceeds in two ways:
- When there is shortage of Wnt ligand, the cytoplasmic β-catenin is gripped by four components which include tumor suppressor Adenomatous Polyposis Coli); GSK3β (glycogen synthase kinase-3β (APC), axin, GSK3β, and casein kinase 1 (CK1). These components form a complex which phosphorylate β-catenin leaving it for proteasomal degradation and inhibiting its nuclear translocation [46].
- While Wnt signaling is active due to the availability of Wnt ligand, the ligand binds to the frizzled receptors and its co-receptor low density lipoprotein-related protein receptor 5/6 (LRP 5/6) to control the level of β-catenin [43]. LRP is phosphorylated by GSK3β and CK1 which than appoints Dishevelled-2 (Dvl2) protein for the inactivation of destruction complex [46]. The amount of β-catenin elevates in the cytoplasm as the outcome of this signaling and later on, is translocated in to the nucleus where it interrelates with T-cell factor (TCF) or lymphoid enhancing factor)VEGF (vascular endothelial growth factor (LEF) to regulate the transcription of target gene [41].
Niclosamide suppresses the Rb by restraining the components like LRP6, Dvl2 and β-catenin of the canonical Wnt/β-catenin pathway inhibiting angiogenesis and inducing cell senescence. Also, combined therapy using niclosamide in conjunction to carboplatin (a chemotherapeutic agent) could be employed to boost its inhibitory potential [41].
Bevacizumab: targeting vascular endothelial growth factor (VEGF)
Angiogenesis is one of the major factors contributing to the complex process of cancer development [47]. Rb has also been characterized as tumor with a compact network of blood vessels [48]. Numerous factors have been proposed to take part in neovascularization of the ocular pathologies, but VEGF and its receptors vascular endothelial growth factor Receptor (VEGFR) are found to be the most prevalent in this regard [49]. Several studies have revealed that the VEGF level elevates in intraocular malignancies [50,51]. On account of these outcomes, anti-VEGF antibodies as a therapeutic agent thought to improve the management of Rb because of clinical indication [52].
Bevacizumab is such an FDA- approved genetically engineered humanized biopharmaceutical (monoclonal antibody) for metastatic colorectal cancer that has the potency to interact with all isoforms of VEGF. Although, bevacizumab has not been approved for Rb; however, modern findings signify its promising therapeutic ability to target VEGF mediated angiogenesis [49]. Bevacizumab not only articulate its effect via constraining neovascularization but also induces modification in vascular function and tumor blood flow [53]. Rb cells undergo differentiation via the expression of vascular endothelial growth factor-2 (VEGFR-2) and a neurotrophin receptor that is activated upon VEGF treatment [54]. Captivatingly, bevacizumab displays its potential by inhibiting the differentiation of Rb cells via hindering the activation of extracellular signal-related kinase ½ (ERK ½) [49]. ERK pathway is found to have the leading role in endothelial cell proliferation in response to VEGF [55]. Additionally, bevacizumab can greatly reduce the development of neurite outgrowths of differentiated Rb as a result of the inhibition of neuro-filament and shank2 expression [49].
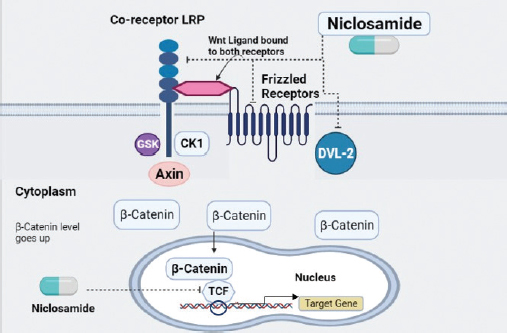
Figure 4. Proposed mechanism of action of niclosamide upon the signaling pathways in Rb.
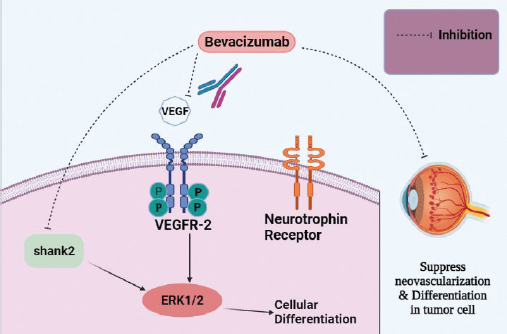
Figure 5. Bevacizumab, a monoclonal antibody, targeting VEGF or its receptors. It also targets shank2 and interferes with its expression.
For the treatment of advance stage retinal carcinoma, bevacizumab is used in conjunction with carboplatin that inhibits ERK 1/2 and Akt pathway inducing cellular apoptosis, cell cycle arrest, and inhibiting angiogenesis [47]. While, bevacizumab alone, only inhibits cellular differentiation though not provoke apoptosis [49].
Aflibercept: Another VEGF Inhibitor
Aflibercept is a potent angiogenic inhibitor, targeting multiple VEGF ligands [56]. It represents a next generation therapeutic agent which is produced by the recombination of a constant region (Fc) of human immunoglobulins G (IgG) antibody amalgamated to portions of immunoglobulins (Ig) i.e., second Ig domain of VEGFR-1 and the third Ig domain of VEGFR-2 [57,58]. Aflibercept works as a decoy receptor and is preferred over bevacizumab in that, it has prominent affinity to interact with VEGF-A compared to bevacizumab and even its natural VEGFR receptors [57]. Aflibercept is an approved drug in USA and Europe for metastatic colorectal cancer. Now, clinical research studies are exploring its use for the management of pediatric retinal cancer [59]. Reviewing the literature, it has been demonstrated that the expression of VEGF protein from its corresponding mRNA mediated Rb cellular proliferation [60]. Aflibercept not only hinders the formation of new vessels in retinal cancer cells but also suppresses the existing vasculature [58]. It also reduces the invasion of tumor to the choroid and represses tumor via targeting VEGF and inducing apoptosis. The decrease in angiogenesis in Rb upon the administration of aflibercept is associated with increase in cellular death analogous to AICAR [61].
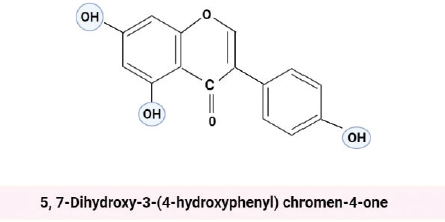
Figure 6. The chemical structure of genistein.
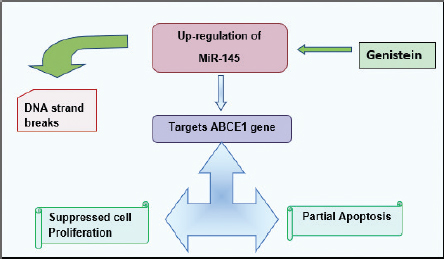
Figure 7. The drawn flow chart shows the proposed mechanism of action of Genistein in Rb. MiR-145 in Rb targets ABCE1 gene causing DNA strand break that induces apoptosis and represses proliferation [65].
Genistein: Up-Regulating miR-145
Genistein, an isoflavone extracted from soy plants, is a biologically active compound with chemical formula 5, 7-Dihydroxy-3-(4-hydroxyphenyl) chromen-4-one [59,62].
The consumption of soy compounds including genistein have been reported in numerous studies having anti-metastatic potential particularly in prostate and breast cancer [63]. Comprehensive studies about the probable mechanism of genistein indicate that it affects most of the proteins concerned with the cancer progression and a potent inhibitor of Nuclear transcription Factor Kappa Beta (NF-κB) and Akt pathways that controls cellular proliferation [64].
Emerging research trends in the management of Rb have depicted clinical potency of genistein. Genistein have the strength to conquer the propagation and dissemination of retinal cancer cells via up-regulating miR-145 [65]. MiR-145 has been defined as the candidate tumor suppressor microRNA and regulates different genes depending upon the type of carcinoma [66]. In Rb, genistein mediated up-regulation of miR-145 induces its inhibitory effect on ABCE1 gene (oncogenic in nature) which evoke cell cycle arrest, induces partial apoptosis and prevent colonization of metastatic retinal cancer [65].
Quercetin: An Anti-Tumor Flavonoids
For cancer medication, light has been shed on the role of anticipated naturally occurring compounds from plants for instance polyphenols. Quercetin is such a plant extract of distinctive flavonoid nature, whose anti-tumor activity has been documented for different types of cancer including lung, prostate, colon cancer etc. [67]. Experimental analyses for the underlying mechanism of quercetin dictate its anti-tumorigenic character via suppression of angiogenesis, proliferation and invasion [68]. In 2017, two researchers along with their co-workers have presented their individual reports exploring the potential of quercetin against Rb.
According to Liu and Zhou, quercetin is capable of suppressing Rb via cell cycle arrest at G1 phase which reduces cell viability. It can also encourage apoptosis by up-regulating mitochondrial membrane potential mediated synthesis of cytochrome c which subsequently activates the caspase-dependent apoptotic pathway [69]. Caspase-9 and caspase-3 play major role in this regard [70]. It has also been observed that quercetin activates p38-mitogen activated protein kinase (p38 MAPK) and c-Jun N-terminal kinase (JNK) signaling pathways that stimulate the caspases to execute apoptosis [69].
In context of the report presented by Song et al. [71], quercetin performs its function in an angiogenic-dependent manner and inhibits VEGFR, reduces cell viability and induces cell death.
Conclusion
Among the pediatric malignancies, Rb tumors have a prominent figure of cases annually. The tumor is marked by multiple symptoms with leukocoria being the chief indication. Rb can protrude to the nervous system resulting in the death of the patient. The survival rate in this type of tumor in very low if the disease is not diagnosed in initial stages. Late diagnosis and low applicability of treatment modalities in the developing and underdeveloped countries leads to poor prognosis. In intense cases, the only treatment option left is enucleation. Literature evaluation provides evidences about angiogenic-dependent progression of Rb. Using traditional chemotherapy produces more toxic effects in pediatric patients as their immune system is not well-matured. So, angiogenesis inhibitors smooth the progress of targeted therapy for Rb tumors. These inhibitors offer the management of the disease with much reduced side effects on healthy cells as most of the compounds used in this regard belongs to natural source such as phyto-extraction based quercetin and genistein. There are also other agents and targets available that can work to inhibit retinal carcinoma in a likely manner that includes glycolytic inhibitors and those that inhibits tumor in hypoxic condition (e.g., gossypol) but are not discussed in this review. To manage such types of tumors, the zone of the focus is the use of combination therapy. Angiogenic-inhibitors in combination with chemotherapy could improve the chances of survival in Rb affected patients. Moreover, targeted therapies like p53, surviving, MiR-200a etc. are also effective in this regard because controlling such targets could greatly reduce tumor invasiveness and metastasis.
Limitations
This paper is a systematic review based on critical analysis of the existing research. This review may provide conjugate data about targets and agents used for Rb management.
Acknowledgement
The authors would like to acknowledge Anhui Agricultural University. We are also wanted to thank Dr. Sidra Hasnain from Superior University, Lahore, Pakistan and Dr. Uqba Mehmood from University of the Punjab, Lahore, Pakistan for their precious comments.
Conflict of interest
None to declare.
Grant support and financial disclosure
None to disclose.
Ethical approval
Not applicable.
Author’s contribution
ZAR: Conceptualization, data collection, writing and editing of original manuscript.
MHZ: Writing of the draft and final editing.
GF: Review of raw data and critical revision of the final manuscript.
ZW: Critical revision and final approval for the manuscript.
ALL AUTHORS: Approval of the final version of the manuscript to be published.
Author details
Zirwa Abdul Rauf1, Muhammad Hamza Zahid1, Feng Guo1, Zaigui Wang1
- College of Life Science, Anhui Agricultural University, Hefei, China
References
- Dimaras H, Corson TW. Retinoblastoma, the visible CNS tumor: a review. J Neurosci Res. 2019;97(1): 29–44. https://doi.org/10.1002/jnr.24213
- Webb AH, Gao BT, Goldsmith ZK, Irvine AS, Saleh N, Lee RP et al. Inhibition of MMP and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer. 2017;17(1):434. https://doi.org/10.1186/s12885-017-3418-y
- Youssef NS, Said AM. Immunohistochemical expression of CD117 and vascular endothelial growth factor in retinoblastoma: possible targets of new therapies. Int J Clin Exp Pathol. 2014;7(9):5725–37.
- Kivelä T. The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93(9):1129–31. https://doi.org/10.1136/bjo.2008.150292
- Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Thériault BL, et al. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol. 2013;14(4):327–34. https://doi.org/10.1016/S1470-2045(13)70045-7
- Bejjani A, Choi MR, Cassidy L, Collins DW, O'Brien JM, Murray T, et al. RB116: an RB1+ retinoblastoma cell line expressing primitive markers. Mol Vis. 2012;8(1):2805–13.
- Athavale V, Khetan V. Kundson to embryo selection: a story of the genetics of retinoblastoma. Taiwan J Opthalmol. 2018;8(1):196–20. https://doi.org/10.4103/tjo.tjo_37_18
- Thériault BL, Dimaras H, Gallie BL, Corson TW. The genomic landscape of retinoblastoma: a review. Clin Experiment Ophthalmol. 2014;42(1):33–52. https://doi.org/10.1111/ceo.12132
- Li J, Zhang Y, Wang X, Zhao R. MicroRNA-497 overexpression decreases proliferation, migration and invasion of human retinoblastoma cells via targeting vascular endothelial growth factor A. Oncol Lett. 2017;13(6):5021–7. https://doi.org/10.3892/ol.2017.6083
- Li Z, Li Q, Wang G, Huang Y, Mao X, Zhang Y, et al. Inhibition of Wnt/β-catenin by anthelmintic drug niclosamide effectively targets growth, survival, and angiogenesis of retinoblastoma. Am J Transl Res. 2017;9(8):3776–86.
- Stenfelt S, Blixt MKE, All-Ericsson C, Hallböök F, Boije H. Heterogeneity in retinoblastoma: a tale of molecules and models. Clin Transl Med. 2017;6(1):42. https://doi.org/10.1186/s40169-017-0173-2
- Gao J, Zeng J, Guo B, He W, Chen J, Lu F, et al. Clinical presentation and treatment outcome of retinoblastoma in children of South Western China. Medicine (Baltimore). 2016;95(42):e5204. https://doi.org/10.1097/MD.0000000000005204
- Reddy SC, Anusya S. Clinical presentation of retinoblastoma in Malaysia: a review of 64 patients. Int J Ophthalmol. 2010;3(1):64–8.
- Abramson DH, Shields CL, Munier FL, Chantada GL. Treatment of retinoblastoma in 2015: agreement and disagreement. JAMA Ophthalmo. 2015;133(11):1341–7. https://doi.org/10.1001/jamaophthalmol.2015.3108
- Dunkel IJ, Shi W, Salvaggio K, Marr BP, Brodie SE, Gobin YP, et al. Risk factors for severe neutropenia following intra-arterial chemotherapy for intra-ocular retinoblastoma. PLoS One. 2014;9(10):e108692. https://doi.org/10.1371/journal.pone.0108692
- Creighton CJ, Fountain MD, Yu Z, Nagaraja AK, Zhu H, Khan M, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res. 2010;70(5):1906–15. https://doi.org/10.1158/0008-5472.CAN-09-3875
- Du M, Wang J, Chen H, Wang S, Chen L, Xu Y, et al. MicroRNA 200a suppresses migration and invasion and enhances the radiosensitivity of NSCLC cells by inhibiting the HGF/c Met signaling pathway. Oncology Rep. 2018;41(1):1497–508. https://doi.org/10.3892/or.2018.6925
- Peng C, Li N, Ng YK, Zhang J, Meier F, Theis FJ, et al. A unilateral negative feedback loop between miR-200 microRNAs and Sox2/E2F3 controls neural progenitor cell-cycle exit and differentiation. J Neurosci. 2012;32(38):13292–308. https://doi.org/10.1523/JNEUROSCI.2124-12.2012
- Montoya V, Fan H, Bryar PJ, Weinstein JL, Mets MB, Feng G, et al. Novel miRNA-31 and miRNA-200a-mediated regulation of retinoblastoma proliferation. PLoS One. 2015;10(9):e0138366. https://doi.org/10.1371/journal.pone.0138366
- Li D, Hu C and Li H: survivin as a novel target protein for reducing the proliferation of cancer cells (Review). Biomed Rep. 2018;8(1):399–406. https://doi.org/10.3892/br.2018.1077
- Garg H, Suri P, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16(1):49. https://doi.org/10.1186/s12935-016-0326-1
- Jaiswal PK, Goel A, Mittal RD. Survivin: a molecular biomarker in cancer. Indian J Med Res. 2015;141(4):389–97. https://doi.org/10.4103/0971-5916.159250
- Shehata HH, Ghalia AHA, Elsayed EK, Ziko OO, Mohamed SS. Detection of survivin protein in aqueous humor and serum of retinoblastoma patients and its clinical significance. Clin Biochem. 2010;43(1):362–6. https://doi.org/10.1016/j.clinbiochem.2009.10.056
- Gibson VP, Hardy P, Chain JL. Turning-off the survivin stimulus: overcoming retinoblastoma drug resistance in vitro. Ann Eye Sci. 2019;4:AB045. https://doi.org/10.21037/aes.2019.AB045
- Ferrario A, Luna M, Rucker N, Wong S, Lederman A, Kim J, et al. Targeting survivin enhances chemosensitivity in retinoblastoma cells and orthotopic tumors. PLoS One. 2016;11(4):e0153011. https://doi.org/10.1371/journal.pone.0153011
- Jeanne A, Schneider C, Martiny L, Dedieu S. Original insights on thrombospondin-1-related antireceptor strategies in cancer. Front Pharmacol. 2015;6(1):252. https://doi.org/10.3389/fphar.2015.00252
- Chen P, Yu N, Zhang Z, Zhang P, Yang Y, Wu N, et al. Thrombospondin-1 might be a therapeutic target to suppress RB cells by regulating the DNA double-strand breaks repair. Oncotarget. 2016;7(5):6105–20. https://doi.org/10.18632/oncotarget.6835
- Xu XL, Singh HP, Wang L, Qi DL, Poulos BK, Abramson DH, et al. Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature. 2014;514(7522):385–8. https://doi.org/10.1038/nature13813
- Laurie NA, Shih CS, Dyer MA. Targeting MDM2 and MDMX in retinoblastoma. Curr Cancer Drug Targets. 2007;7(7): 689–95. https://doi.org/10.2174/156800907782418266
- Qi DL, Cobrinik D. MDM2 but not MDM4 promotes retinoblastoma cell proliferation through p53-independent regulation of MYCN translation. Oncogene. 2017;36(13):1760–9. https://doi.org/10.1038/onc.2016.350
- Folkman J. Introduction. Cancer Metastasis Rev. 1990;9(3):171–4. https://doi.org/10.1007/BF00046358
- Garcia JR, Gombos DS, Prospero CM, Ganapathy A, Penland RL, Chevez-Barrios P. Expression of angiogenic factors in invasive retinoblastoma tumors is associated with increase in tumor cells expressing stem cell marker Sox2. Arch Pathol Lab Med. 2015;139(1):1531–8. https://doi.org/10.5858/arpa.2014-0262-OA
- Baumann P, Mandl-Weber S, Emmerich B, Straka C, Schmidmaier R. Activation of adenosine monophosphate activated protein kinase inhibits growth of multiple myeloma cells. Exp Cell Res. 2007;313(16):3592–603. https://doi.org/10.1016/j.yexcr.2007.06.020
- Rehman G, Shehzad A, Khan AL, Hamayun, M. Role of AMP-activated protein kinase in cancer therapy. Arch Pharm (Weinheim). 2014;347(7):457–68. https://doi.org/10.1002/ardp.201300402
- Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–99. https://doi.org/10.1016/j.ccr.2004.06.007
- Theodoropoulou S, Kolovou PE, Morizane Y, Kayama M, Nicolaou F, Miller JW, et al. Retinoblastoma cells are inhibited by aminoimidazole carboxamide ribonucleotide (AICAR) partially through activation of AMP-dependent kinase. FASEB J. 2010;24(8):2620–30. https://doi.org/10.1096/fj.09-152546
- Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci. 2011;36(9):470–7. https://doi.org/10.1016/j.tibs.2011.06.004
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009;8(1):2385–98. https://doi.org/10.4161/cc.8.15.9082
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–75. https://doi.org/10.1038/nrc2676
- Theodoropoulou S, Brodowska K, Kayama M, Morizane Y, Miller JW, Gragoudas ES, et al. Aminoimidazole carboxamide ribonucleotide (AICAR) inhibits the growth of retinoblastoma in vivo by decreasing angiogenesis and inducing apoptosis. PLoS One. 2013;8(1):e52852. https://doi.org/10.1371/journal.pone.0052852
- Li Y, Li PK, Roberts MJ, Arend R, Samant RS, Buchsbaum DJ. Multi-targeted therapy of cancer by niclosamide: a new application for an old drug. Cancer Lett. 2014;349(1):8–14. https://doi.org/10.1016/j.canlet.2014.04.003
- Satoh K, Zhang L, Zhang Y, Chelluri R, Boufraqech M, Nilubol N, et al. Identification of niclosamide as a novel anticancer agent for adrenocortical carcinoma. Clin Cancer Res. 2016;22(14):3458–66. https://doi.org/10.1158/1078-0432.CCR-15-2256
- Pan JX, Ding K, Wang CY. Niclosamide, an old anti-helminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells. Chin. J. Cancer. 2012;31(4):178–84. https://doi.org/10.5732/cjc.011.10290
- Arend RC, Londoño-Joshi AI, Gangrade A, Katre AA, Kurpad C, Li Y, et al. Niclosamide and its analogs are potent inhibitors of Wnt/β-catenin, mTOR and STAT3 signaling in ovarian cancer. Oncotarget. 2016;7(52):86803–15. https://doi.org/10.18632/oncotarget.13466
- Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31(12):2737–46. https://doi.org/10.1038/emboj.2012.126
- Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(1):1461–73. https://doi.org/10.1038/onc.2016.304
- Zhang Q, Cheng Y, Huang L, Bai Y, Liang J, Li X. Inhibitory effect of carboplatin in combination with bevacizumab on human retinoblastoma in an in vitro and in vivo model. Oncol Lett. 2017;14(5):5326–32. https://doi.org/10.3892/ol.2017.6827
- Lee SY, Kim DK, Cho JH, Koh JY, Yoon YH. Inhibitory effect of bevacizumab on the angiogenesis and growth of retinoblastoma. Arch Ophthalmol. 2008;126(7):953–8. https://doi.org/10.1001/archopht.126.7.953
- Heo JW, Kim JH, Cho CS, Jun HO, Kim DH, Yu YS, et al. Inhibitory activity of bevacizumab to differentiation of retinoblastoma cells. PLoS One. 2012;7(3):e33456. https://doi.org/10.1371/journal.pone.0033456
- Missotten GS, Schlingemann RO, Jager MJ. Angiogenesis and vascular endothelial growth factors in intraocular tumors. Dev Ophthalmol. 2010;46(1):123–32. https://doi.org/10.1159/000320015
- Cheng Y, Zheng S, Pan CT, Yuan M, Chang L, Yao Y, et al. Analysis of aqueous humor concentrations of cytokines in retinoblastoma. PLoS One. 2017;12(5):e0177337. https://doi.org/10.1371/journal.pone.0177337
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(1):363–72. https://doi.org/10.1016/j.ophtha.2005.11.019
- Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33(5 Suppl 10):S1–7. https://doi.org/10.1053/j.seminoncol.2006.08.002
- Kim JH, Kim JH, Kim DH, Cho CS, Jun HO, Yu YS, et al. Neurotrophin receptors TrkA and TrkB in retinoblastoma are differentially expressed depending on cellular differentiation. Tumour Biol. 2009;30(5–6):233–41. https://doi.org/10.1159/000243766
- Srinivasan R, Zabuawala T, Huang H, Zhang J, Gulati P, Fernandez S, et al. Erk1 and Erk2 regulate endothelial cell proliferation and migration during mouse embryonic angiogenesis. PLoS One. 2009;4(12):e8283. https://doi.org/10.1371/journal.pone.0008283
- Chau CH, Figg WD. Aflibercept in pediatric solid tumors: moving beyond the trap. Clin Cancer Res. 2012;18(18):4868–71. https://doi.org/10.1158/1078-0432.CCR-12-2212
- Ciombor KK, Berlin J. Aflibercept--a decoy VEGF receptor. Curr Oncol Rep. 2014;16(2):368. https://doi.org/10.1007/s11912-013-0368-7
- Ricci V, Ronzoni M, Fabozzi T. Aflibercept a new target therapy in cancer treatment: a review. Crit Rev Oncol Hematol. 2015;96(3):569–76. https://doi.org/10.1016/j.critrevonc.2015.07.001
- Kim SH, Kim CW, Jeon SY, Go RE, Hwang KA, Choi KC. Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Lab Anim Res. 2014;30(4):143–50. https://doi.org/10.5625/lar.2014.30.4.143
- Areán C, Orellana ME, Abourbih D, Abreu C, Pifano I, Burnier MN. Expression of vascular endothelial growth factor in retinoblastoma. Arch Ophthalmol. 2010;128(2):223–9. https://doi.org/10.1001/archophthalmol.2009.386
- Kim DY, Choi JA, Koh JY, Yoon YH. Efficacy and safety of aflibercept in in vitro and in vivo models of retinoblastoma. J Exp Clin Cancer Res. 2016;35(1):171. https://doi.org/10.1186/s13046-016-0451-7
- Ganai AA, Farooqi H. Bioactivity of genistein: a review of in vitro and in vivo studies. Biomed Pharmacother. 2015;76(1):30–8. https://doi.org/10.1016/j.biopha.2015.10.026
- Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–82. https://doi.org/10.1007/s10555-010-9238-z
- Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269(2):226–42. https://doi.org/10.1016/j.canlet.2008.03.052
- Wei D, Yang L, Lv B, Chen L. Genistein suppresses retinoblastoma cell viability and growth and induces apoptosis by upregulating miR-145 and inhibiting its target ABCE1. Mol Vis. 2017;23(1):385–94.
- Yang XW, Zhang LJ, Huang XH, Chen LZ, Su Q, Zeng WT, et al. miR-145 suppresses cell invasion in hepatocellular carcinoma cells: miR-145 targets ADAM17. Hepatol Res. 2013;4(5):551–9. https://doi.org/10.1111/hepr.12152
- Slusarz A, Shenouda NS, Sakla MS, Drenkhahn SK, Narula AS, Macdonald RS, et al. Common botanical compounds inhibit the hedgehog signaling pathway in prostate cancer. Cancer Res. 2010;70(8):3382–90. https://doi.org/10.1158/0008-5472.CAN-09-3012
- Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One. 2012;7(10):e47516. https://doi.org/10.1371/journal.pone.0047516
- Liu H, Zhou M. Antitumor effect of quercetin on Y79 retinoblastoma cells via activation of JNK and p38 MAPK pathways. BMC Complement Altern Med. 2017;17(1):531. https://doi.org/10.1186/s12906-017-2023-6
- Huang YC, Kuo CL, Lu KW, Lin JJ, Yang JL, Wu RS, et al. 18α-Glycyrrhetinic acid induces apoptosis of HL-60 human leukemia cells through caspases- and mitochondria-dependent signaling pathways. Molecules. 2016;21(7):872. https://doi.org/10.3390/molecules21070872
- Song W, Zhao X, Xu J, Zhang H. Quercetin inhibits angiogenesis-mediated human retinoblastoma growth by targeting vascular endothelial growth factor receptor. Oncol Lett. 2017;14(3):3343–8. https://doi.org/10.3892/ol.2017.6623
Keywords: Retinoblastoma, p53 activation, Angiogenesis inhibitors, Signaling pathways.
Publication History
Received: January 05, 2021
Revised: March 31, 2021
Accepted: June 01, 2021
Published: June 30, 2021
Authors
Zirwa Abdul Rauf
College of Life Science, Anhui Agricultural University, Hefei - China.
Muhammad Hamza Zahid
College of Life Science, Anhui Agricultural University, Hefei - China.
Feng Guo
College of Life Science, Anhui Agricultural University, Hefei - China.
Zaigui Wang
College of Life Science, Anhui Agricultural University, Hefei - China.
